Chemical Reaction
Chemical Reactions Examples
A chemical reaction in chemistry is a process that transforms one or more substances or reactants to form new types of substances...
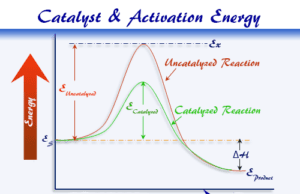
Chemical Catalyst
Catalyst in Chemistry
Chemical catalyst or simply catalyst is a foreign substance that increases the speed or rate of the reaction by lowering activation energy...
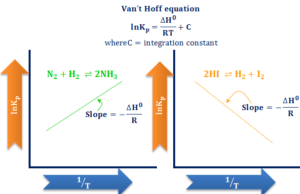
Van’t Hoff Equation
Van't Hoff Equation-Effect of Temperature on Equilibrium Constant
Van't Hoff equation connecting chemical equilibrium constant (Keq) and temperature by thermodynamics relation of Gibbs-Helmholtz free energy...
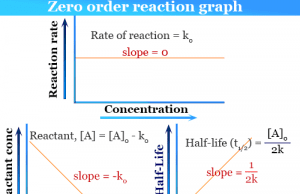
Zero Order Reaction
Zero Order Reaction Kinetics
Zero order reaction kinetics in chemistry define the rate of chemical reaction in terms of reactant and product per unit time....
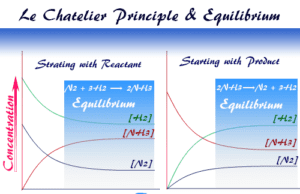
Le Chatelier Principle
Chemical Equilibrium and Le Chatelier's Principle
Le Chatelier's principle in chemistry predicts the effect on the system at chemical equilibrium when some of the factors...
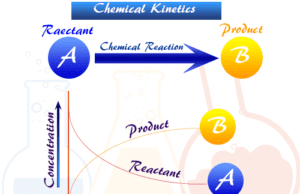
Chemical Kinetics
Chemical Kinetics in Chemistry
Chemical kinetics is the branch of physical chemistry concerned with the rate change of concentration of reactants and products of chemical...
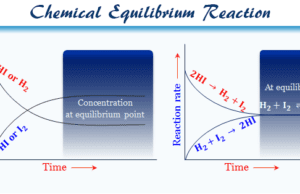
Chemical Equilibrium
Chemical Equilibrium Definition
Chemical equilibrium in chemistry is the dynamic state of the system in which the concentration of reactants and products has no further...
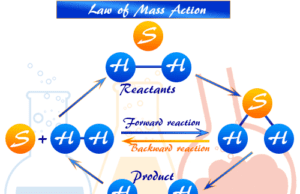
Law Mass Action
Law of Mass Action in Chemistry
Law of Mass Action or Mass Action Law formula in chemical equilibrium was first developed by two Norwegian chemists,...