Dipole Moment of Molecules
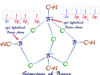
Dipole moment molecules benzene and substituted benzene (xylene) in chemistry can be calculated by different theoretical or experimental formula. For example, the dipole moment of the benzene molecule is zero suggesting that the benzene ring has a regular hexagonal planner aromatic structure with zero resultant bond moment. All the carbon atoms in benzene molecules contain sp2 hybridized atomic orbitals. Therefore, the chemical bond in the benzene molecule has double bond properties with five canonical structures having different bond energy. The aromatic hydrocarbon benzene was first isolated by Faraday in 1825 in cylinders of the compressed illuminating gas molecule from the pyrolysis of whale oil. In learning chemistry, substituted benzene molecule also shows aromatic properties with (4n + 2)π electrons.
Dipole Moment of Benzene Derivatives
If a hydrogen atom in the benzene ring is substituted by another atom or group, this molecule acquires bond polarity. Examples of such benzene derivatives which show dipole moment are chlorobenzene, nitrobenzene, phenol, benzyl alcohol, etc.
The dipole moment of o, p, m-isomers of the benzene ring can be calculated from the formula,
μ2 = m12 + m22 + 2 m1m2 cosθ.
Ortho Para Meta Position in Benzene
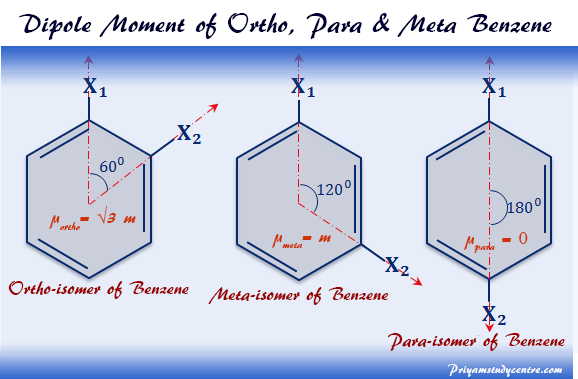
When one group or element is introduced into the ring, only one product is produced. The ortho, para, meta, or o, p, m isomers are formed by the introduction second group in the benzene ring given above the picture.
The dipole moment of the ortho isomer of the benzene molecule will have the highest value and the p isomer has the lowest value equal to zero like methane.
Dipole Moment Formula
The resultant dipole moment of molecules is equal to the vectorial sum of individual bonds or group moments. It depends on the structure of the molecules.
The dipole moment of the di-substituted molecules like xylene, dichlorobenzene, and dinitrobenzene can be calculated by the vectorial addition formula,
μ2 = m12 + m22 + 2 m1m2 cosθ
Where m1 and m2 are individual group moments
θ = angle at which m1 and m2 are inclined to each other
Dipole Moment of Benzene Derivatives
Ortho, para, and meta-xylene are the substituted derivatives of benzene having the molecular formula C6H4(CH3)2.
Dipole Moment of o-xylene
For the o-xylene molecule, the groups moment of CH3 group = m and θ = 60°.
From the dipole moment formula,
μ2o-xylene = 2m2(1 + cos60°)
= 3m2
Therefore, the dipole moment of o-xylene = √3m
p-xylene Dipole Moment
For the o-xylene molecule, θ = 180°.
From the dipole moment formula,
μ2p-xylene = 2m2(1 + cos180°)
= 0
Therefore, the dipole moment of o-xylene = 0
Dipole Moment of m-xylene
For the o-xylene molecule, θ = 120°.
From the dipole moment formula,
μ2p-xylene = 2m2(1 + cos120°)
= m2
Therefore, the dipole moment of o-xylene = m. From the above three calculations, the dipole moment di-substituted molecules of a benzene ring or xylene, μortho > μmeta >μpara.
Similar way, we can calculate the dipole moment of o, p, and m-dinitrobenzene or dichlorobenzene.
Dipole Moment of 1, 3, 5 trinitrobenzene
1, 3, 5 trinitrobenzene is a symmetrical non-polar molecule. When it is dissolved in benzene, a charge transfer bonding involves some kind of donner acceptor interaction.
Due to the formation of charge-transfer complexes in the C6H6 solution, the compound shows a significant dipole moment.
Problem: If the dipole moment of the o-xylene molecule = 0.693D, calculate the μ for the toluene molecule.
Answer: For o-xylene, θ = 60° and μ = √3 m.
∴ mxylene = 0.693/√3
= 0.4D
Again for toluene, θ = 120°
∴ μtoluene = 0.4D
Problem: Why p-dinitrobenzene has μ = 0 but para – dihydroxybenzene μ ≠ 0?
Answer: In p-dinitrobenzene, the substituted nitro group is on the same plane of the benzene ring but in p-dihydroxybenzene, the substituted hydroxy groups are not on the same plane.
Among these two substituted molecules, the dipole moment of p-dinitrobenzene is zero but the p-dihydroxybenzene molecule is polar.