Methanol (Methyl Alcohol)
Methanol or methyl alcohol also called carbinol is a colourless, inflammable, poisonous alcohol with the chemical formula CH3OH. It is missable with water in all proportions and also missable with most organic solvents. Methyl alcohol is a very useful solvent for paints, varnishes, shellac, etc. In the earliest time, it was obtained from wood. Destructive distillation of wood gives tar and pyroligneous acid. Pyroligneous acid contains three organic compounds such as methanol, acetone, and acetic acid. Therefore, it was also called the wood spirit. Methanol burns in the air to form a faintly luminous flame. Methanol vapour forms an explosive mixture with air or oxygen when ignited.
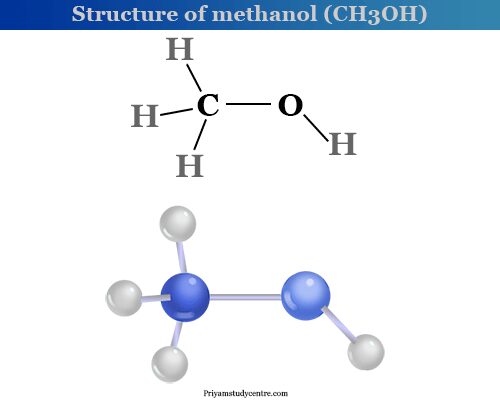
Structure of Methanol
Molecular weight determination and analysis show that the molecular formula of methanol is CH4O. Therefore, methanol contains quadrivalent carbon, bivalent oxygen, and univalent hydrogen in its structure.
- Only one hydrogen atom in methyl alcohol is replaceable by a sodium atom. It is suggested that the one hydrogen atom in methyl alcohol is different from the other three hydrogen atoms.
- However, the presence of the hydroxyl group is confirmed by the reaction between methyl alcohol and phosphorus pentachloride. Methyl chloride, hydrogen chloride, and phosphoryl chloride are formed by this reaction.
- Infrared spectroscopic studies show the absorption band of hydroxyl compounds in the region of 3650 – 3580 cm−1. It is true if there is no hydrogen bonding. Intermolecular hydrogen bonding produces the absorption band in the region of 3550 – 3230 cm−1.
Chemical Properties
Methanol is colourless, poisonous, and volatile liquid that burns in the air with a faintly luminous flame. It forms an explosive mixture with air or oxygen. It has a methyl group linked with the hydroxyl group. Some important properties of methanol are given below in the table,
| Methanol |
||
| IUPAC name | Methanol | |
| Other names | Carbinol Hydroxymethane Methyl alcohol Methyl hydroxide Methylic alcohol Methylol Pyroligneous spirit Wood alcohol Wood spirit |
|
| Chemical formula | CH4O or CH3OH | |
| Molar mass | 32.04 g mol−1 | |
| Appearance | Colourless liquid | |
| odor | Sweet and pungent | |
| Density | 0.792 g/cm3 | |
| Vapor pressure | 13.02 kPa at 20 °C | |
| Solubility | Soluble in water and most organic solvents | |
| Melting point | − 97.6 °C | |
| Boling point | 64.7 °C | |
| Acidity (pka) | 15.5 | |
| Conjugate acid base pair | acid | base |
| methyloxonium | methanolate | |
| Viscosity | 0.545 mPa s at 25 °C | |
| Dipole moment | 1.69 Debye | |
| CAS number | 67-56-1 | |
Methanol Production
Production from Synthesis Gas
Methanol is produced from synthesis gas at 200 atmosphere pressures over a chemical catalyst containing oxides of copper, zinc, and chromium at 300 °C.
CO + 2 H2 → CH3OH
The production of methanol is 100 percent if the proper precaution is taken. By changing the catalyst and ratio of carbon monoxide to hydrogen, the product is a variety of higher alcohol.
Production of Methanol from Methane
Catalytic oxidation of methane produced CH3OH. A mixture of methane and oxygen (9:1 ratio) produced methyl alcohol when passed through a copper tube at 200 °C and 100 atmospheric pressure.
CH4 + ½ O2 → CH3OH
Uses of Methanol
Methyl alcohol is a type of alcohol produced mostly from natural gas. The most common use of methyl alcohol may include,
- It is the base material for the production of acetic acid and formaldehyde.
- It is also used for the production of hydrocarbons such as ethylene and propylene.
- Methyl alcohol and its derivatives like formaldehyde and acetic acid are used for the production of acrylic plastic, synthetic fabrics, and fibers that are used in clothing.
- It is used as a solvent for paints, varnishes, shellac, etc.
- For the production of pharmaceuticals, agrichemicals, and perfumes, we also used widely methyl alcohol.
- It is an efficient energy carrier for factories and different types of energy generation processes.
- It is used as a fuel because it is easier to store than hydrogen and natural gas. It is a biodegradable and renewable source of energy.
- Methanol is also used for making methylated spirit and automobile antifreezing mixtures.