Phenolphthalein Indicator
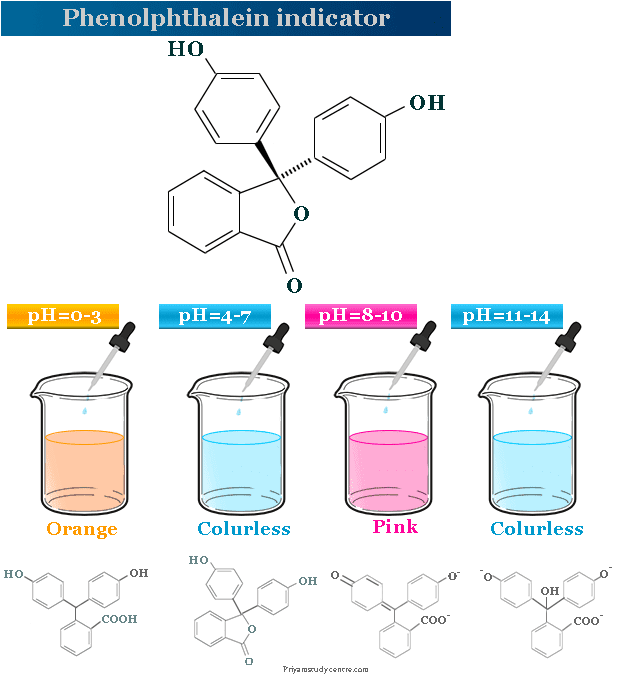
Phenolphthalein indicator which we used in acid-base titration is an organic compound with the formula C20H14O4. It is a white crystalline solid powder that is insoluble in water solution but soluble in alkali to form pink colour. The colour of the phenolphthalein solution depends upon the pH range of the solution. It is a triphenylmethane derivative prepared from phenol and phthalic anhydride. It is a dye of the phthalein group. Phenolphthalein is majorly used as an indicator rather than a dye.
Phenolphthalein Solution
In the presence of the alkali pink colour of phenolphthalein is developed due to dianion formation. The dianion has canonical forms with a quinonoid structure in one of the rings.
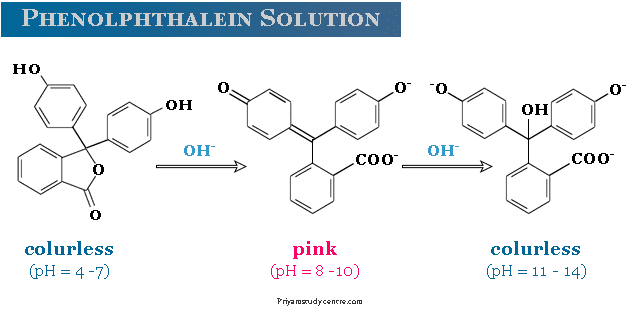
However, in the presence of excess strong alkali, the solution of phenolphthalein becomes colurless. The colurless solution arises due to the destruction of the quinonoid structure by the nucleophilic attack of the hydroxyl group.
Properties
It is a white crystalline solid powder that is soluble in water solution. It has four different forms according to the pH scale range of the aqueous solution.
- Under a strongly acidic solution (pH = 0 – 3), it protonated to form an orange color.
- Between strongly acidic and slightly basic conditions (pH = 4 – 7), it is colorless.
- The most popular pink dianion form is obtained in the presence of an alkali (pH = 8 – 10).
- However, in the presence of a strong alkali (pH range 11 – 14), the solution of phenolphthalein is colorless.
Some common properties are given below the table,
| Properties | |
| IUPAC name | 3,3-Bis(4-hydroxyphenyl)isobenzofuran-1(3H)-one |
| Chemical formula | C20H14O4 |
| Molar mass | 318.328 g mol-1 |
| Appearance | White crystalline powder |
| Density | 1.277 g/cm3 at 32 °C |
| Melting point | 258 to 263 °C |
| Solubility in water | 400 mg/L |
| Solubility in other solvents | very soluble in ethanol and ether |
| λmax in ultraviolet-visible spectroscopy | 1st: 552 nm 2nd: 374 nm |
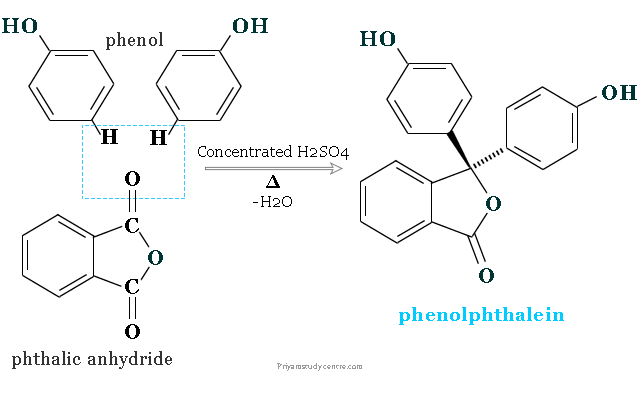
Synthesis
It is prepared by condensing phthalic anhydride (1 mole) with phenol (2 moles) in the presence of concentrated sulfuric acid.
Uses of Phenolphthalein
- Phenolphthalein is used as an indicator very widely in acid-base titrations rather than a dye. Like methyl red, bromothymol blue, and thymol blue, it is also a universal indicator.
- It is used in chemistry classes for the study of chemical kinetics.
- It is used as a component of disappearing inks or disappearing dye in toys. We used a mixture of phenolphthalein and sodium hydroxide in disappearing inks. It reacts with carbon dioxide in the air and the pink color disappears.
- A reduced form of phenolphthalein dye is used to identify specific substances in blood by the Kastle–Meyer test.
- In medicine, it has been used as a laxative for a long time. Phenolphthalein is removed from laxative products due to its carcinogenic properties.