Carbon Dioxide Gas
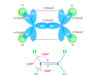
Carbon dioxide (chemical formula CO2) is a colorless, orderless gas that can be easily liquefied under critical temperature and pressure. It is the most important industrial chemical or greenhouse gas that causes global warming in our environment. The carbon dioxide molecule is linear and symmetrical with a C=O chemical bond length of 116.3 pm. The green plants absorb atmospheric CO2 for the production of carbohydrates through the process of photosynthesis. It is a major industrial chemical obtained from several industrial processes. For example, CO2 is formed in fermentation industries, calcination of limestone, and flue gases from coal-based power plants. Carbon dioxide gas is fairly soluble in water by forming a weakly acidic solution of H3CO3.
Reducing Carbon Dioxide in the Atmosphere
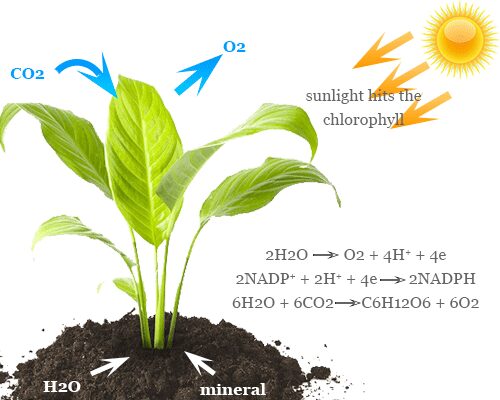
Atmospheric CO2 is the main source of carbon in our life. The major consumers of CO2 gas from the atmosphere are green plants and oceans. Green plants generally reducing CO2 from the atmosphere to prepare carbohydrates through the process of photosynthesis. While oceans dissolve CO2 to form carbonate rocks.
Today, with the increased burning of carbonaceous fuels and decreases in forest areas, the concentration of atmospheric CO2 has been at an alarming stage. Therefore, it causes to rise in the global temperature of our earth’s environment. The effect of rising global temperature is called the greenhouse effect. Carbon dioxide molecules in the atmosphere trap the infrared electromagnetic radiation coming from the earth’s surface.
Structure of Carbon Dioxide
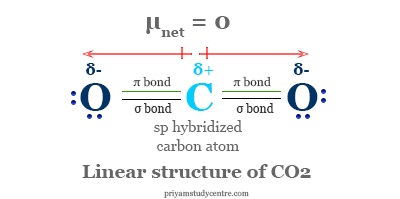
The carbon atom in a linear CO2 molecule is sp hybridized. Two half-filled sp hybridized carbon atoms form two sigma bonds with two oxygen atoms. Similarly, two singly occupied p-orbitals of carbon atom formed two pi-bonds each of the p-orbital of two oxygen atoms. Therefore, the linear CO2 molecule has two sigma and two pi bonds in its structure.
Physical Properties
CO2 is a colorless, orderless gas and fairly soluble in water. The solubility increases remarkably under pressure.
| Properties of carbon dioxide | |||
| Chemical formula | CO2 | ||
| Molar mass | 44.009 g/mol | ||
| Appearance | Colorless gas | ||
| Density | Gas | Liquid | Solid |
| 1562 kg/m3 at 1 atm and −78.5 °C | 1101 kg/m3 at −37 °C | 1.977 kg/m3 at 1 atm and 0 °C | |
| Critical Constants | Critical temperature (TC) | Critical pressure (PC) | |
| 30.978 °C | 72.808 atm | ||
| Dipole moment | 0 Debye | ||
| Molecular shape | Linear | ||
| Heat capacity | 37.135 J K−1 mol−1 | ||
Facts About CO2
Carbon dioxide is mild Lewis acid by the formation of carbonates with oxide or hydroxide ions. It also acts as a Lewis base toward metal atoms in a low oxidation state. Neutralization of the dissolved CO2 occurs by two routes depending upon the pH scale of the solution.
pH < 8, the overall reaction follows pseudo-first-order chemical kinetics since H2O is always in large excess.
H2O + CO2 → H2CO3 (slow)
H2CO3 + OH− → HCO3− + H2O (fast)
pH > 10, the overall rate is first order. Between pH 8 to 10, all the chemical equilibrium is involved.
CO2 + OH− → HCO3− (slow)
HCO3− + OH− → CO3−2 + H2O (fast)
Chemical Reaction
CO2 reduces electrochemically to form carbon monoxide (CO) in an aqueous medium.
CO2 + 2 H+ + 2 e → CO + H2O
Electrolysis by platinized titanium oxide (TiO2) or transition metal complexes generally reduces CO2 to various products like carbon monoxide, methane, methyl alcohol, etc.
The reduction of CO2 to organic compounds under ordinary conditions occurs during photosynthesis.
6 CO2 + 6 H2O → C6H12O6 + 6 O2
Uses of Carbon Dioxide
- Liquid carbon dioxide is used in the refrigeration of ice cream, meat, fish, etc. Over the last two centuries, compressed liquid CO2 has been gradually replaced by dry ice due to lower production costs and transport facilities.
- Liquid CO2 is also used as a substitute for chlorofluorocarbons in aerosol propellants.
- Gaseous carbon dioxide is extensively used to prepare soda water and other soft drinks preparation
- It also provides an inert atmosphere for welding and neutralizes the alkali.
- In the manufacture of sodium carbonate, sodium bicarbonate, and urea, we used a huge quantity of gaseous carbon dioxide.