How to Calculate pH and pOH of a Solution?
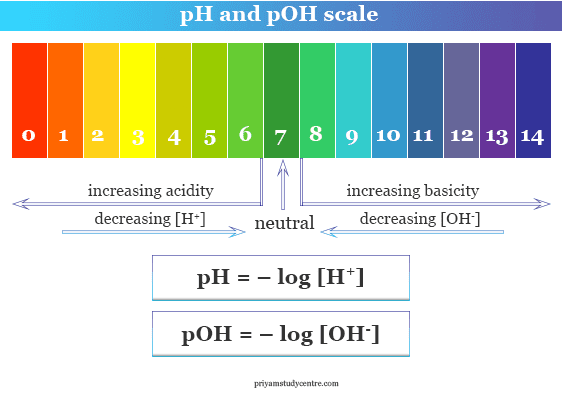
pH and pOH scale define and measure the acidity and basicity of neutral, acidic, and basic solutions in water based on the relative concentration of hydrogen and hydroxyl ions mainly in chemistry, biology, and soil science. The terms pH and pOH scale are widely used and important for chemical, biological, and soil health measurement by translating the values of the concentration of hydrogen or hydroxyl ions or using pH paper and meter. Sorensen first invented or used the term known as pH scale or chart for measuring the concentration of hydrogen and hydroxyl ions for acid and alkaline solutions.
What is pH definition?
According to Sorensen’s definition, pH (full form potential of hydrogen) is negative of the logarithm of the activity or concentration of hydrogen ion.
pH = − log aH+
When the solution is very dilute, aH+ = CH+
∴ pH = − log CH+
The Sorensen definition is largely used in chemistry, biology, and agronomy for the simple mathematical calculation of pH and pOH scale values. Technically we use instruments like pH paper and pH meter for acid and base determination.
What is pOH in chemistry?
In learning chemistry, the concentration value of hydroxyl ion represents the term known as the pOH in place of pH.
pOH = − log (COH−)
- If the acidity of a solution goes down by 100 fold its pH scale range goes up by two units.
- In the same way, when hydroxyl ion will go down by two units means pOH values go from 13 to 11.
pH scale range
pH scale range or concentration of H+ ion differentiate neutral, acidic, and basic solutions.
The acidity or basicity level defines the pH and pOH scale chart value of the neutral, acidic, or alkaline solution in chemistry.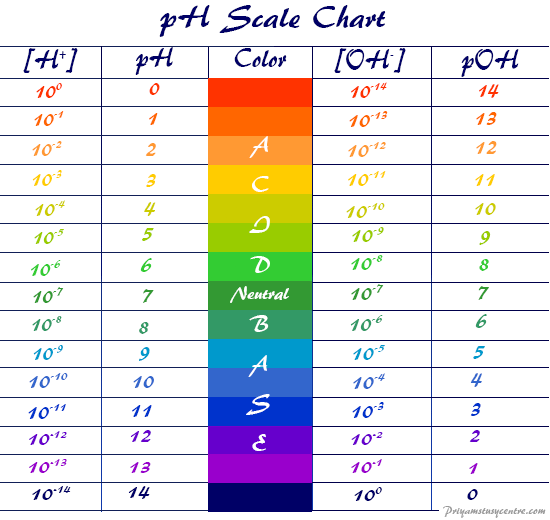
Acidic, basic, and neutral solutions
| The concentration of H+ in a solution | pH | The concentration of OH− in a Solution | pOH |
| 100 | 0 | 10−14 | 14 |
| 10−1 | 1 | 10−13 | 13 |
| 10−2 | 2 | 10−12 | 12 |
| 10−3 | 3 | 10−11 | 11 |
| 10−4 | 4 | 10−10 | 10 |
| 10−5 | 5 | 10−9 | 9 |
| 10−6 | 6 | 10−8 | 8 |
| 10−7 | 7 | 10−7 | 7 |
| 10−8 | 8 | 10−6 | 6 |
| 10−9 | 9 | 10−5 | 5 |
| 10−10 | 10 | 10−4 | 4 |
| 10−11 | 11 | 10−3 | 3 |
| 10−12 | 12 | 10−2 | 2 |
| 10−13 | 13 | 10−1 | 1 |
| 10−14 | 14 | 100 | 0 |
pH value of a neutral solution
A neutral solution or pure water solution is a solution where the concentrations of hydrogen and hydroxyl ions are equal or the pH values of acids and bases are equal.
Therefore, CH+ = COH− = 10−7 M
From the definition, we can write simply,
pH = pOH = 7
Therefore, this scale range or level explains that the neutral water solution has the same value of pH and pOH which stands for 7.
pH scale for acids
An acidic solution has a hydrogen ion greater than a hydroxyl ion.
∴ CH+ > COH−
CH+ > 10−7
COH− < 10−7
From the definition, pH < 7 and pOH > 7.
From the above equation, an acidic pure water solution pH scale range stands from zero to less than seven, and the pOH range stands from greater than seven to fourteen.
pH range for bases
In an alkaline or basic solution, the concentration of hydrogen ion is less than that of hydroxyl ion or CH+ < COH−. Therefore, the measured pH range is greater than 7 to 14.
Ionic product of water
Water molecules ionize weakly to form conjugate acid-base pairs like hydrogen ion and hydroxyl ion in solution. There will always be a chemical equilibrium between hydrogen and hydroxyl ions in the water solution.
H2O ⇄ H+ + OH−
Dissociation of water will have its equilibrium constant value,
k × CH2O = CH+ × COH−
where CH2O, CH⁺, and COH− = concentrations of water, hydrogen, and hydroxyl ions.
But in any dilute aqueous solution, the concentration of water = 55.5 moles/liter, can be taken as a constant.
Therefore, k × CH₂O = Kw = CH+ × COH−
where Kw = ionic product of water
Chemical reactions that absorb a specific heat by the system from the surroundings are known as endothermic reactions in thermodynamics.
A specific heat or energy (13.7 kcal) is absorbed from the surroundings for the ionization of water. Therefore, according to the Le Chatelier principle, increasing temperature will facilitate higher values of the dissociation constant (Kw).
The ionic product of hydrogen ion and hydroxyl ion has a constant having a value of 10−14. Therefore, from the Sorensen pH and pOH definition in chemistry,
pH + pOH = 14
What is the ph of water?
The concentration of hydrogen and hydroxyl ion or pH scale in pure water is equal to the ionic product of water. The ionic product of pure water = 10−14 and the concentration of hydrogen ion and hydroxyl ion are the same.
∴ Kw = CH+ × COH−
=10−7 × 10−7
= 10−14
The above facts describe the pure water solution pH scale level. The pH value for pure is equal to 7 because CH+ = COH− = 10−7.
This equation shows that the concentration of hydrogen ions and hydroxyl ions in water is inversely proportional to each other. To maintain constant Kw, if hydrogen ion concentration increases 100 fold then hydroxyl ion decrease 100 fold.
Measurement of pH
For 0.01 M sulfuric acid
Sulfuric acid is a dibasic acid. Normality of sulfuric acid = 2 × molarity of the sulfuric acid solution. The values of 0.1 m and 0.2 N sulfuric acids are the same.
Therefore, the pH measurement value for 0.1 M or 0.2 N sulfuric acid,
= − log (0.2)
= 0.699
For 0.02 M hydrochloric acid
Hydrochloric acid is a strong electrolyte and is completely dissociated in the solution.
Therefore the measured pH value of 0.02 M hydrochloric acid (HCl) solution,
= − log (2×10−3)
= (3 − log2)
∴ Calculated pH of 0.2 M HCl = 2.7
For 0.02 M acetic acid
Acetic acid is a weak organic acid and the concentration of hydrogen ion in 0.02 M acetic acid.
Therefore, the measured pH value for 0.002 acetic acid,
= − log (CH+)
= − log (2 × 10−4)
∴ Calculated pH of CH3COOH = 3.7
Negative pH scale
Nitric acid, sulfuric acid, and hydrochloric acid in pure water solution are completely dissociated and regarded as almost equally strong acids.
However, the mathematical definition or application of the pH meter provides the negative scale value when the concentration of hydrogen ion in some content exceeds 1 g equivalent. Therefore, measuring pH and pOH scales value for such polar acid, base, or salt solutions is avoided because these solutions are not likely to dissociate fully.
The absolute concentration of such a strong acid solution is measured in terms of relative molarity rather than the potential of hydrogen or pH scale value.