What is formic acid?
Formic acid or methanoic acid is a colourless, pungent, corrosive liquid or simplest carboxylic acid with the chemical formula H2CO2 or HCOOH. It is missable in all proportions with water and ethanol due to the formation of hydrogen bonding with water or ethanol molecule.
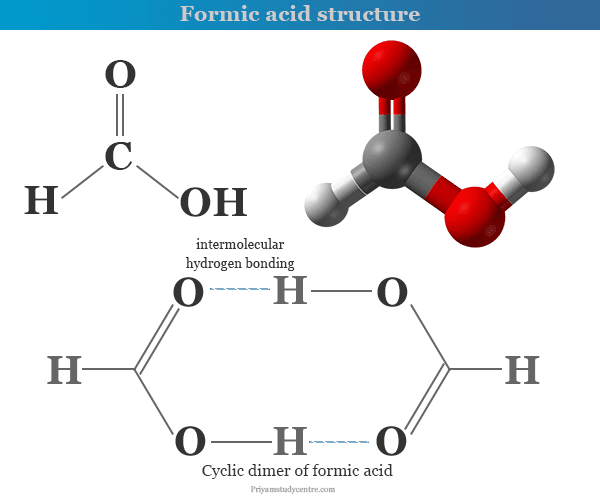
The gaseous formic acid does not obey ideal gas law due to the formation of hydrogen bonding. It is a stronger acid than any monocarboxylic acid like acetic acid.
It occurs naturally in the venom of bees and ants. Formic acid is used mainly for preservation and antibacterial agent in livestock feed.
Industrially, formic acid preparation is done by heating sodium hydroxide with carbon monoxide at 210 °C and 6 to 10 atmosphere pressure.
Formic acid preparation
How formic acid is prepared in lab?
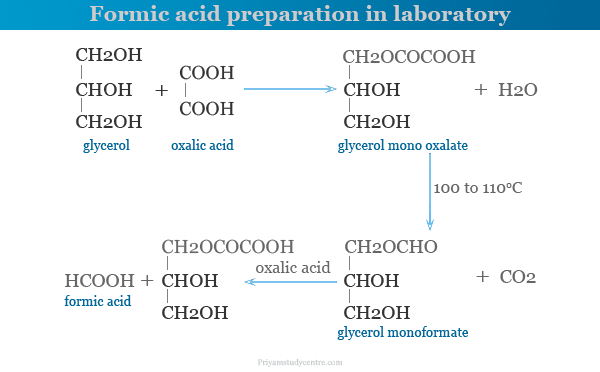
- The most important laboratory preparation of formic acid, to heating glycerol with oxalic acid at 100 to 110 °C.
- Glycerol monoxalate is formed by this reaction.
- It decomposes to form glycerol monoformate and carbon dioxide.
- When the evolution of carbon dioxide stops, more oxalic acid is added for the production of HCOOH.
- The distillate contains formic acid and water.
The aqueous formic acid solution cannot be fractionalized to give anhydrous acid due to the boiling point of the acid (100.5 °C). Therefore, an aqueous solution can be neutralized with lead carbonate.
Concentrate the solution until lead formate is crystallizing. The precipitate is dried and heated at 100 °C in a current of hydrogen sulfide.
(HCO2)2Pb + H2S → 2 HCO2H + PbS
The anhydrous acid produced by this method contains a small amount of hydrogen sulfide. It can be removed by adding dry lead tetraformate and redistilling.
Industrial Production
It is prepared industrially by heating sodium hydroxide and carbon monoxide under 6 to 10 atmosphere pressure and 210 °C temperature.
NaOH + CO → HCOONa
An aqueous solution of acid is obtained by distilling the sodium salt with dilute sulfuric acid. Anhydrous formic acid is obtained from the aqueous solution by the addition of butyl formate followed by distillation.
Chemical Properties
Formic acid differs from the other member of the monocarboxylic acid series by its powerful reducing properties.
- It reduces ammonical silver nitrate and the salts of many heavy metals.
- It converts mercuric chloride into mercurous chloride.
Methanoic acid is far less volatile than is to be expected from its molecular weight due to the formation of intermolecular hydrogen bonding. It exists as a dimer in vapour phase and aqueous solution. In the liquid phase, it exists as a polymer.
| Formic acid | ||
| IUPAC name | Methanoic acid | |
| Chemical formula | CH2O2 or HCOOH | |
| Molar mass | 46.025 g mol−1 | |
| Molecular shape | Planar | |
| Appearance | Colurless fuming liquid | |
| Odor | pungent | |
| Density | 1.220 g/mL | |
| Melting point | 8.4 °C | |
| Boiling point | 100.8 °C | |
| Solubility | Miscible in water and most of the organic solvents like ether, acetone, ethyl acetate, glycerol, methanol, ethanol, etc. | |
| Vapor pressure | 35 mmHg at 20 °C | |
| Acidity (pka) | 3.745 | |
| Conjugate base | Formate | |
| Dipole moment | 1.41 D (gas) | |
| CAS Number | 64-18-6 | |
Chemical reaction
It can be dehydrated to carbon monoxide by concentrated sulfuric acid. When formic acid is heated under pressure at 160 °C temperature, it decomposes to form carbon dioxide and hydrogen.
CO + H2O ← HCOOH → CO2 + H2
The same decomposition takes place at room temperature in presence of chemical catalyst like platinum, iridium, rhodium, etc.
When metallic formates are heated with sodium hydroxide, hydrogen gas is evolved.
HCOONa + NaOH → H2 + Na2CO3
Calcium or zinc formate is strongly heated to form formaldehyde.
(HCOO)2Ca → HCHO + CaCO3
When sodium and potassium formate is rapidly heated to 360 °C, hydrogen and oxalate are formed.
2 HCO2Na → (COONa)2 + H2
Methanoic acid is different from other monocarboxylic acids such as acetic acid due to the participation of addition reactions with alkenes. It reacts with alkenes to form formate ester. Under suitable conditions, it adds to alkenes to form a larger carboxylic acid.
Formic acid uses
- Formic acid is a useful organic synthetic reagent that is used mainly as a preservative and antibacterial agent in livestock feed.
- It is a useful material in the leather and textile industries. Therefore, large consumption is used for the production of lather and dyeing or finishing textile.
- Some formate esters are useful materials for artificial flavoring and the production of perfumes.
- It is too much stronger than acetic and is used as a volatile pH scale modifier in capillary electrophoresis.
- In the fuel cells, it can be used directly for the production of fuel cells and indirectly for the production of hydrogen fuel cells.
- Formic acid is used in different types of cleaning products like limescale remover and toilet bowl cleaner.