Ethylene Oxide Gas
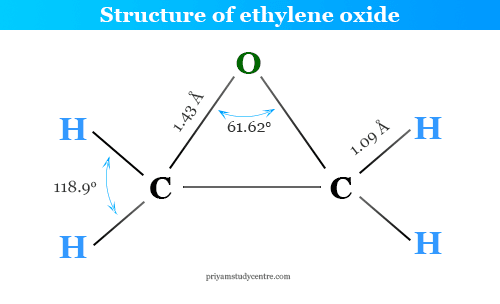
Ethylene oxide gas (EtO) is a cyclic ether or simple organic epoxide compound with the chemical formula C2H4O. French chemist Charles-Adolphe Wurtz first prepared ethylene oxide by treating 2-chloroethanol with potassium hydroxide in 1859. In ethylene oxide structure, an oxygen atom is linked to the two carbon atoms with a three-member cyclic ring. At room temperature, ethylene oxide is a colourless gas that transforms into liquid at 0 °C. EtO is isomeric with acetaldehyde (CH3CHO) and vinyl alcohol (CH2=CH−OH). The viscosity of liquid ethylene oxide is much lower than that of water. It is readily miscible in water, ethanol, diethyl ether, and many organic solvents. Industrially, it has been produced by passing ethylene and air under pressure over a silver catalyst at 200 to 400 °C.
Due to the hazardous nature of gaseous ethylene oxide, we commonly handle and ship it as a refrigerated liquid. A large volume of EtO can be used for making many consumer products and non-consumer chemicals such as detergents, thickeners, solvents, plastics, and various organic chemicals (ethylene glycol, ethanolamines, polyglycol ethers, cellosolve, etc). It can also be used for the sterilization of medical equipment.
Properties of Ethylene Oxide
At room temperature, EtO is a highly flammable, extremely explosive, carcinogenic, irritating, and anesthetic gas. The chemical formula of the ethylene oxide molecule is C2H4O which is isomeric with acetaldehyde (CH3CHO) and vinyl alcohol (CH2=CH−OH).
According to the IUPAC system of nomenclature, EtO is denoted by the prefix epoxy. Therefore, EtO is called epoxyethane. It is named oxiran due to the presence of the oxiran ring. Oxiran compounds are also referred to as cyclic ethers or alkenes oxides.
| Properties | |
| Chemical formula | C2H4O |
| CAS Number | 75-21-8 |
| IUPAC name | Oxirane Epoxyethane Oxacyclopropane |
| Molar mass | 44.052 g/mol |
| Density | 0.8821 g/cm3 |
| Appearance | A colourless gas that has a characteristic sweet odor of ether |
| Melting point | −112.46 °C |
| Boiling point | 10.4 °C |
| Solubility in water | Miscible in all proportion |
| Dipole moment | 1.94 D |
| Heat capacity | 47.9 J mol−1 K−1 |
| Main hazards | Highly flammable, extremely explosive, carcinogenic, irritating, and anesthetic gas |
The C−O group stretching in epoxides absorbs in the regions 1260−1240 cm−1 but in acyclic ethers containing two alkyl groups absorbs in the regions 1150−1060 cm−1. Therefore, such data can easily distinguish epoxy compounds from ethers.
Ethylene Oxide Production
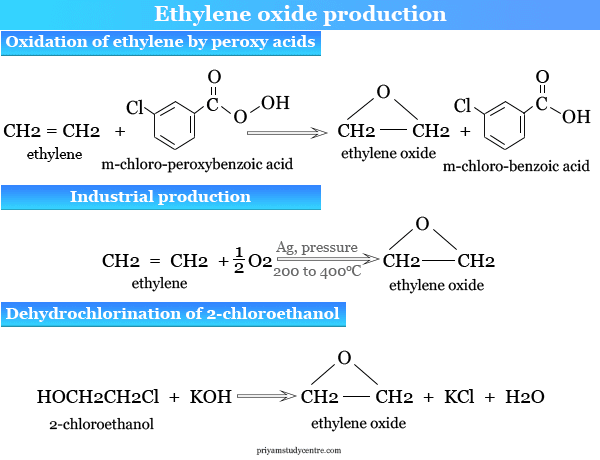
French chemist Charles-Adolphe Wurtz first prepared it by treating 2-chloroethanol (chlorohydrin) with potassium hydroxide in 1859.
Dehydrochlorination of 2-Chloroethanol
Ethylene oxide was produced in the 19th century by the chlorohydrin process where ethylene chlorohydrin is prepared by reacting ethylene with hypochlorous acid (chlorine in water).
Cl2 + H2O → HOCl (hypochlorous acid) + HCl
CH2=CH2 + HOCl → HO–CH2CH2–Cl (chlorohydrin)
Ethylene chlorohydrin can be converted to ethylene oxide by treating it with sodium hydroxide or potassium hydroxide, calcium hydroxide, barium hydroxide, magnesium hydroxide, or carbonates of alkali metals or alkaline earth metals.
2 HOCH2CH2Cl + Ca(OH)2 → 2 (CH2CH2)O + CaCl2 + 2H2O
Such a method is no longer used in industry because most of the chlorine that was used was lost and unwanted organochlorine by-products were generated.
Therefore, this process has been gradually replaced by various other processes which can be described below:
Ethylene Oxide from Ethylene
Alkenes like ethylene can be converted to ethylene oxide by epoxidation with peroxy acids such as peroxybenzoic acid (C6H5COO2H), meta-chloro-peroxybenzoic acid, monoperoxyphthalic acid, and p-nitro-peroxybezoic acid.
CH2=CH2 + C6H5COO2NH → (CH2CH2)2O + C6H5COONH
Peroxy trifluoroacetic acid (CF3COO2H) is a very good reagent for epoxidation and hydroxylation which converts the double bond of ethylene into an epoxide.
Industrial production
Ethylene oxide is manufactured by passing ethylene and air under pressure over a chemical catalyst like silver at 200 to 400 °C.
CH2=CH2 + O2 → 2 (CH2CH2)O
Chemical Reaction of Ethylene Oxide
Ethylene oxide can readily react with various inorganic and organic compounds by opening its ring. It commonly participates in chemical reactions with nucleophiles via the SN2 mechanism in acidic and alkaline media.
Molecular Rearrangement
Ethylene oxide undergoes molecular rearrangement on heating at about 400 °C in the presence of a catalyst (Al2O3, H3PO4) to form its isomer acetaldehyde (CH3CHO).
(CH2CH2)O + heat → CH3CHO
Addition of Water and Alcohol
An aqueous solution of ethylene oxide is stable and does not participate in any noticeable chemical changes. However, at room temperature when adding a small amount of strongly diluted sulfuric acid or any other acids, it immediately transforms into ethylene glycol.
(CH2CH2)O + H2O → HO–CH2CH2–OH
Such type of chemical reaction can also occur in the gas phase in the presence of a catalyst (salt of phosphoric acid). When we use an alkaline catalyst, it can converted to polyethylene glycol.
n (CH2CH2)O + H2O → HO–(–CH2CH2–O–)n–H (polyethylene glycol)
Ethylene oxide can produce mono-ethers when reacts with alcohol in the presence of a small amount of acid as a catalyst. For example, it is produced ethyl cellosolve (2-ethoxyethanol) when reacts with ethanol.
(CH2CH2)O + C2H5OH → HO–CH2CH2–OC2H5
It can also react with alcohol under the influence of a basic catalyst. The reaction with fatty alcohols (lauryl, stearyl, and oleyl alcohols) can proceed in the presence of sodium metal, sodium hydroxide, or boron trifluoride.
Halide Addition Reaction
Gaseous ethylene oxide readily reacts with aqueous solutions of haloacids (HCl, HBr, HI) to form halohydrins. Such reactions always produce by-product ethylene glycol with a mixture of diethylene glycol. For pure products, the reaction can be conducted in the gas phase or an organic solvent.
(CH2CH2)O + HCl → HO–CH2CH2–Cl
Similarly, the addition of hydrogen cyanide produced ethylene cyanohydrin.
(CH2CH2)O + HCN → HO–CH2CH2–CN
Reaction with Ammonia
Ethylene oxide reacts with ammonia to form a mixture of three amino alcohols. They are usually called ethanolamines.
C2H4O + NH3 → HOCH2CH2NH2
HOCH2CH2NH2 + C2H4O → (HOCH2CH2−)2NH
(HOCH2CH2−)2NH + C2H4O → (HOCH2CH2−)3N
A similar reaction can proceed when reacting with primary or secondary amines. C2H4O forms choline when reacts with trimethylamine in the presence of water.
C2H4O + (CH3)3N + H2O → [HOCH2CH2N (CH3)3]+OH−
Reduction Reaction
Ethylene oxide is a symmetrical epoxide that forms ethyl alcohol when hydrogenated or reduced in the presence of nickel, platinum, palladium, boranes, lithium aluminum hydride, etc.
(CH2CH2)O + H2 → 2 CH3CH2OH
Other unsymmetrical epoxides can give more highly substituted alcohol.
Propylene oxide → CH3CHOHCH3 + CH3CH2CH2OH
Epoxides are readily reduced to the corresponding alkenes by t-phosphines or zinc dust and acetic acid. For example, ethylene oxide can give ethene when reduced by zinc dust and acetic acid.
(CH2CH2)O + H2 → CH2=CH2 + H2O
Dimerization
In the presence of dilute sulfuric acid, it dimerizes to form dioxan. Dioxan is a useful solvent for cryoscopic and ebullioscopy work.
The dimerization reaction of ethylene oxide is unselective and it produces acetaldehyde due to isomerization. Therefore, various catalysts such as platinum, platinum-palladium, or iodine with sulfolane can be used to increase selectivity and the speed of dimerization.
Methyl Cellosolve
Methyl cellosolve or monomethyl ether is prepared by heating EtO with methanol under pressure.
C2H4O + CH3OH → HO–CH2CH2–OCH3
Cellosolves are very useful solvents since they contain both alcohol and ether functional groups.
Ethylene oxide to Ethylene Glycol
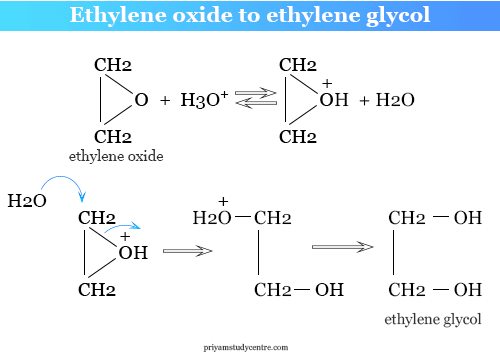
Aqueous solutions of ethylene oxide are stable and exist for a long time without any chemical reaction but it is converted into ethylene glycol when added small amount of dilute sulfuric acid.
(CH2CH2)O + H3O+ → HO−CH2CH2−OH + H+
The mechanism of the reaction in acid media follows the steps given in the picture,
Ethylene Oxide Uses
The direct use of ethylene oxide gas is very low due to health hazards and reactivity. It is readily flammable in the air. Therefore, EtO is used for making many consumer products and non-consumer chemicals such as detergents, thickeners, solvents, plastics, and various organic chemicals (ethylene glycol, ethanolamines, polyglycol ethers, etc).
- Ethylene oxide is the most impotent intermediate used for the production of different types of industrial organic compounds.
- It is used for the production of ethylene glycol.
- When EtO is heated with methanol under pressure, the monomethyl ether of glycol or methyl cellosolve is formed. The corresponding ethyl ether is known as ethyl cellosolve. Cellosolves are very useful solvents due to the presence of alcohol and ether functional groups.
- Directly, EtO is used as a sterilizing agent, disinfecting agent, and fumigant. Therefore, it is used for gas-phase sterilization of medical equipment and instruments, packaging materials and clothing, and surgical and scientific equipment.
- EtO gas reacts with ammonia to form a mixture of three amino alcohols which are usually referred to as ethanolamines. It is widely used for the preparation of emulsion.
- It is used as a fungicide. Ethylene oxide gas can also be used to accelerate the maturation of tobacco leaves.