Formaldehyde Formula and Structure
Formaldehyde or methanal is a colorless, pungent smelling gas or organic compound with the chemical formula HCHO. It is a powerful disinfectant or antiseptic which is extremely soluble in water. Formaldehyde uses mainly for the manufacture of dyes, resins, and plastics. It is prepared industrially by air oxidation of methanol in the presence of a silver catalyst at 400 °C while pure aqueous formaldehyde solution may be obtained by refluxing paraformaldehyde with water and stored as a formalin solution. A very low level of formaldehyde molecule is found in most living organisms. Due to its carcinogenic properties, formaldehyde can have adverse effects on our health. The chemical formula, Lewis structure, and hybridization of the formaldehyde molecule are given below the picture,
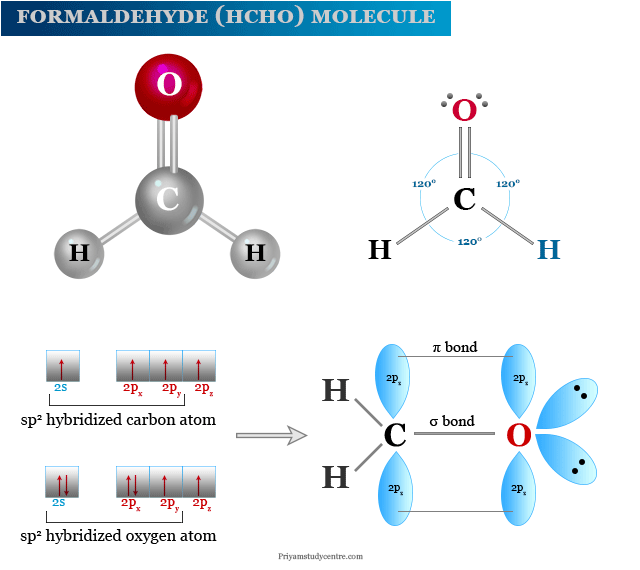
Structure of Formaldehyde
Methanal is a simple aldehyde that contains carbon, hydrogen, and oxygen in its structure.
The carbon and oxygen atoms in the formaldehyde molecule are sp2 hybridized. The sp2 hybridized carbon atom is attached to two hydrogen atoms by a single chemical bonding and bonded to the oxygen atom by one sigma and one pi bond or a double bond.
Paraformaldehyde
A white crystalline solid paraformaldehyde (melting point 121 to 123 °C) is obtained by drying the formaldehyde solution. Paraformaldehyde reforms formaldehyde when heated.
The chemical formula of paraformaldehyde is HO(CH2O)nH, where n has values between 6 to 50. It is a form of formaldehyde polymer.
Uses of Formaldehyde
- In chemical industries, the colorless pungent-smelling formaldehyde uses mainly for the manufacture of dyes, gelation, resins, and plastics.
- It can be used for the production of various chemicals such as formaldehyde resins, polyoxymethylene plastics, 1,4-butanediol, and methylene diphenyl diisocyanate.
- It is dissolved in water to form formalin solutions. It is used widely for the preservation of food products and biological specimens like tissue or cells.
- Formaldehyde is a powerful antiseptic or disinfectant because it kills many types of bacteria and fungi.
- It can be used for the treatment of urinary infections.
- It is used for manufacturing several types of wooden products such as furniture, cabinetry, moldings, flooring, shelving, etc.
- It is used for the manufacturing of vaccines, and personal care products such as sanitizers, paper towels, napkins, etc.
Due to its carcinogenic properties, the European Union has banned the use of formaldehyde in personal care products.
Chemical Properties
The colorless, pungent smelling aldehyde like formaldehyde is highly soluble in water. A saturated formalin solution contains 40 percent of methanal, 8 percent of methanol, and 52 percent of water.
| Formaldehyde | |
| IUPAC name | Methanal |
| Other names | Methyl aldehyde Methylene oxide Formalin (aqueous solution) Carbonyl hydride |
| Chemical formula | CH2O or HCHO |
| Molecular structure | Trigonal planar |
| Molar mass | 30.026 g mol−1 |
| Appearance | Colorless gas |
| Density | 0.8153 g/cm3 at − 20 °C |
| Melting point | − 92 °C |
| Boiling point | − 21 °C |
| Solubility in water | 400 g/L |
| Vapor pressure | Less than 1 atm |
| Acidity (pKa) | 13.27 |
| Dipole moment | 2.33 Debye |
| Heat capacity | 35.387 J mol−1 K−1 |
| Standard molar entropy | 218.76 J mol−1 K−1 |
| CAS number | 50-00-0 |
It is a useful disinfectant that kills several types of bacteria and fungi.
Formaldehyde undergoes many general reactions of aldehydes but differs in reaction with ammonia. It does not form an aldehyde-ammonia compound but gives hexamethylenetetramine (melting point 260 °C). The hexamethylenetetramine contains three fused chairs in its structure.
6 HCHO + 4 NH3 → (CH2)6N4 + 6 H2O
Reaction of Formaldehyde
It has no alpha-hydrogen. Therefore, it participates in the Cannizzaro reaction in which two molecules of HCHO are involved. One molecule is converted into methanol and the other is converted into sodium salt of formic acid.
2 HCHO + NaOH → HCOONa + CH3OH
It can also participate in the crossed Cannizzaro reaction. The nature of the final product depends on the structure of another aldehyde. For example, benzaldehyde forms benzyl alcohol.
In a dilute aqueous solution, it can be 100 percent dehydrated to form methylene glycol with the molecular formula CH2(OH)2. It is another form of HCHO.
HCHO + H2O → CH2(OH)2
It can be treated with concentrated sulfuric acid to form polyoxymethylenes, (CH2O)n, H2O, where n is greater than 100. Polyoxymethylenes are white solids that are insoluble in water and reform HCHO when heated.
Formaldehyde gas is slowly polymerized to give trioxymethylene, metaformaldehyde, or 1,3,5-trioxane.
Metaformaldehyde or 1,3,5-Trioxane is a white solid with the molecular formula (CH2O)3. It can be prepared by distilling a methanal solution with a small amount of sulfuric acid.
Effects of Formaldehyde
It is found naturally in our environment. About 90 percent of HCHO is obtained in the upper atmosphere. It is also formed in the environment by the combustion or oxidation of methane and other hydrocarbons in the presence of sunlight.
It is formed from different construction materials effects our environment and causes air pollution. Therefore, the high level of formaldehyde has adverse effects on human health. It causes eyes, nose, skin irritation, burning sensation in the throat, headaches, and difficulty in breathing.
In 2011, the US National Toxicology Program suggested that formaldehyde is a human carcinogen.
A low level of HCHO is found in most living organisms. It is an intermediate that is formed in the metabolism of amino acids.
It can be naturally present in fruits and vegetables like pear, apple, green onion, etc. Meats, Bombay duck, codfish, crustaceans, and dried mushrooms are other common sources of formaldehyde.
Production of Formaldehyde
It was first prepared in 1859 by the Russian chemist Aleksandr Butlerov but he cannot identify the gas.
It was first identified by August Wilhelm von Hofmann. He produced formaldehyde by dehydrogenation or air oxidation of methanol vapor in the presence of a silver catalyst. Present-day it is the most common industrial route for the production of HCHO.
In industry, it is produced by catalytic oxidation of methanol. We used catalysts like platinum, silver, molybdenum, and vanadium pentoxide for the oxidation of methanol.
2 CH3OH + O2 → 2 CH2O + 2 H2O
CH3OH → CH2O + H2
It can also be produced by the air oxidation of methane from natural gas in the presence of various metallic oxides. This route of production of formaldehyde is not used industrially because methanol is more easily oxidized than methane.