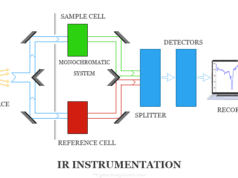
Ultraviolet Visible (uv vis) Spectroscopy
Ultraviolet visible spectroscopy or uv vis spectroscopy involves the measurement of absorption or transmittance of energy in the ultraviolet and visible regions of the electromagnetic spectrum. The ultraviolet visible spectroscopy principle or theory is used mainly to measure the absorption peaks (λmax) and molar absorptivity of multiple chemical bonding in organic compounds. However, ultraviolet visible (uv vis) light in absorption spectroscopy is also used to determine the colour of transition metals. On passing electromagnetic radiation in the ultraviolet and visible regions through a compound with multiple bonds. However, a portion of the radiation is normally absorbed by the compound. The amount of absorption depends on the wavelength of the radiation and the structure of the molecules. The absorption of radiation is due to the transition of electrons from lower energy orbital to higher energy orbitals.
The absorption of organic compounds and transition metals is processed in two stages.
- In the first stage, M + hν → M∗. Ultraviolet visible spectroscopy involves the excitation of the species by absorption of photons with a limited lifetime.
- In the second stage, M∗ converts to a new species by photochemical reaction.
Absorption in the uv visible region leads to the excitation of bonding electrons. Therefore, the absorption peaks can be correlated with the kind of bond that exists in the species. Hence absorption spectroscopy in the ultraviolet visible region is valuable for the characterization of functional groups in organic molecules.
Principle of Ultraviolet Visible Spectroscopy
The UV Visible spectroscopy principle is based on the absorption of ultraviolet light or visible light by chemical compounds. To occur a chemical reaction, the reacting molecules must be activated by acquiring activation energy.
In photochemical reactions, the activation energy is obtained by absorption of radiation or light energy from uv visible spectroscopy. In order to study the mechanism of the photochemical reaction, we need to study the following types of electronic transition:
- Sigma to sigma star transition (σ → σ∗)
- n to sigma star transition (n → σ∗)
- n to pi star transition (n → π∗)
- Pi to pi star transition (π → π∗)
Absorption Peaks
Absorption peaks (λmax) and molar absorptivity (∈max) and possible electronic transition of some common compounds are given below the table,
| Compounds | λmax (nm) | ∈max (L/cm/mol) | Electronic transition |
| Alkenes | 177 | 1.3 × 104 | n → π∗ |
| Alkyne | 178 – 225 | 10 × 103 – 150 | n → π∗ |
| Carbonyl | 186 – 280 | 1.0 × 103 – 16 | n → π∗ |
| Carboxyl | 204 | 41 | n → π∗ |
| Amide | 214 | 60 | n → π∗ |
| Azo | 339 | 5 | n → π∗ |
| Nitro | 280 | 22 | n → π∗ |
| Ketone | 282 | 27 | n → π∗ |
| Water | 167 | 1.48 × 103 | n → σ∗ |
| Methyl alcohol | 184 | 15 | n → σ∗ |
| Benzene | 204 | 7.9 × 103 | n → σ∗ |
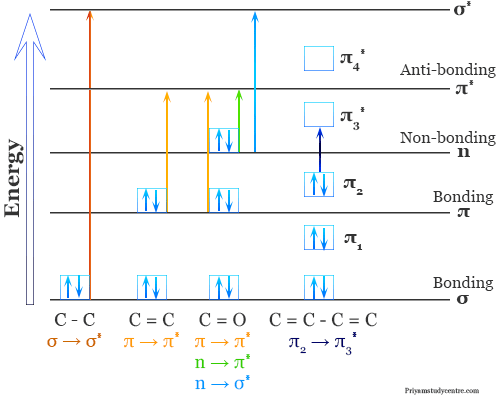
Sigma to Sigma Star Transition (σ → σ∗)
A transition of an electron from a bonding sigma orbital to a higher energy antibonding sigma orbital is designated σ → σ∗ (sigma to sigma star transition in ultraviolet visible spectroscopy).
In alkanes, there are only sigma bonds are available. Therefore, alkenes are showing this type of transition. In general, sigma bonds are very strong. Therefore, high energy is required for σ → σ∗ transition.
n to sigma star transition (n → σ∗)
n to sigma star transition (n → σ∗) involves saturated compounds with one hetero atom like oxygen, nitrogen, fluorine, chlorine, etc. Normally, saturated halides, alcohols, ethers, aldehydes, ketones, and amines participate in this type of transition.
These transitions require comparatively less energy than the σ → σ∗ transition. In saturated alkyl halides, the energy required for n to sigma star transition (n → σ∗) decreases with the increase in the size of the halogen atom or decrease in electronegativity of the atom.
Due to the greater electronegativity of chlorine than iodine, the n electron on the chlorine atom is comparatively difficult to excite. The n electrons on the iodine atom are loosely bound.
Pi to Pi Star Transition (π → π∗)
Pi to pi star transition (π → π∗) in uv vis absorption spectroscopy is available in compounds with unsaturated centers like unsaturated hydrocarbons and carbonyl compounds.
It requires lesser energy than n to sigma star transition (n → σ∗). In simple alkenes several transitions are available but the n → π∗ transition requires the lowest energy.
n to pi Star Transition (n → π∗)
In n to pi star transition (n → π∗), an electron in an unshared pair on a hetero atom is excited to π∗ antibonding orbital. It involves the least amount of energy of all types of transition in ultraviolet visible spectroscopy. Therefore, the n → π∗ transition gives the absorption with a longer wavelength.
In saturated ketones, n → π∗ transitions around 280 nm are the lowest energy transition. n → π∗ is forbidden by symmetry consideration. Thus the intensity of the band due to this transition is low, although the wavelength is long.
Applications of UV Visible Spectroscopy
- Ultraviolet (200 to 400 nm) and visible spectroscopy (400 to 750 nm) are used to detect functional groups and the nature of unsaturation in organic chemistry. The longer the conjugation give the maximum wavelength of absorption spectrum in organic compounds. A compound with sufficient conjugation becomes coloured. For example, lycopene is a compound that gives red colour due to eleven conjugated double bonds.
- Generally, electronic spectroscopy can differentiate conjugated diene from non-conjugated dienes and conjugated diene from conjugated trienes.
- The study of ultraviolet spectroscopy can be used to identify suitable tautomeric species.
- It is used to know the extent of strain organic molecules like 2-substituted diphenyl or acetophenones.
- The absorbed spectrum of d block or transition metals is an ultraviolet visible region. For f block elements transitions are possible due to f electrons. The inner transition metals have narrow peaks due to shielding electrons. On the other hand, in the case of the first and second transition series broad peaks are seen in the visible region. Spectral properties and colour of transition metal ions involve electronic transition among different energy levels of d-orbitals.
- In all the charge transfer metal complexes, the metals serve as electron pair acceptors. The concentration of a colour solution can find out by the absorption of light. In the visible region of the spectrum, it is possible to determine concentration by three techniques, calorimetry, photometric photometry, and spectrophotometry. Spectrophotometry is useful for determining absorbance in both ultraviolet and visible regions.
- In suitable cases, ultraviolet visible (uv vis) spectroscopy is used to determine reaction rates and pKa values in chemistry.