Phenol Molecular Formula
Phenol is a colorless aromatic organic compound with the molecular formula C6H5OH. It is moderately soluble in cold water but solubility increases in ethanol or ether solution due to the presence of a benzene ring. Phenol is used as an antiseptic and disinfectant. It is also used for the production of dyes, drugs, and phenol-formaldehyde resin or bakelite. It can be extracted first from coal tar but today it can be extracted commercially by the cumene phenol process. The structure of phenol contains one hydroxyl group directly attached to the benzene ring. It is a crystalline solid which turns pink on exposure to air or light. It behaves like a weak acid due to losing one hydrogen ion to form a phenoxide anion. The acidity of phenol can be explained by the stability or resonance of phenoxide ions.
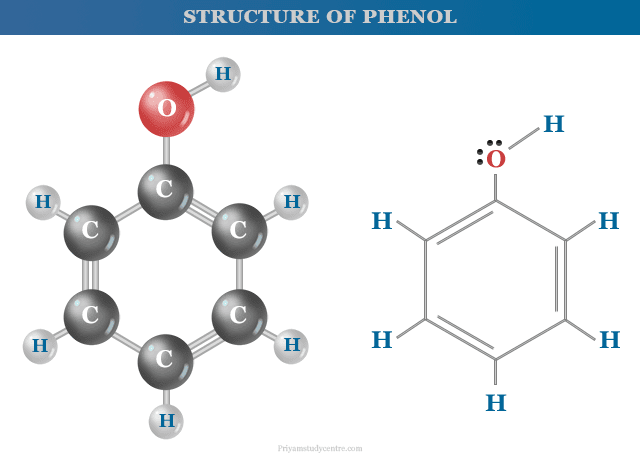
Structure of Phenol
Phenol is an organic compound with the molecular formula C6H5OH. Therefore, the structure of phenol contains a benzene ring which is directly attached to a hydroxyl group.
Properties of Phenol
It is a monohydric aromatic alcohol that is moderately soluble in cold water but readily soluble in ethanol or ether. It is a stronger acid than alcohol due to the resonance structure or formation of a phenoxide anion.
The infrared absorption region of the phenolic hydroxyl group is the same as that of the alcoholic hydroxyl group. They can be differentiated by their nucleus which has its own absorption spectrum.
| Phenol | |
| IUPAC name | benzenol |
| Other names | Phenic acid Carbolic acid Phenylic acid Hydroxybenzene |
| Chemical formula | C6H6O |
| Molar mass | 94.113 g/mol |
| Appearance | colorless crystalline solid |
| Density | 1.07 g/cm3 |
| Melting point | 40.5 °C |
| Boiling point | 181.7 °C |
| Conjugate base | Phenoxide ion (C6H5O−) |
| Dipole moment | 1.224 D |
| Solubility in water | 8.3 g/100 mL |
| CAS Number | 108-95-2 |
Boiling Point
Like other alcohols, C2H5OH molecules are associated through intermolecular hydrogen bonding which extends over a chain. Therefore, the boiling point of phenol is much higher than the expected value obtained from their molecular weight.
Acidity of Phenol
The acidity of phenol is weaker than carboxylic acids but stronger than alcohols. Due to resonance, the oxygen atom of the hydroxyl group acquires a positive charge. Therefore, it can facilitate the release of the proton.
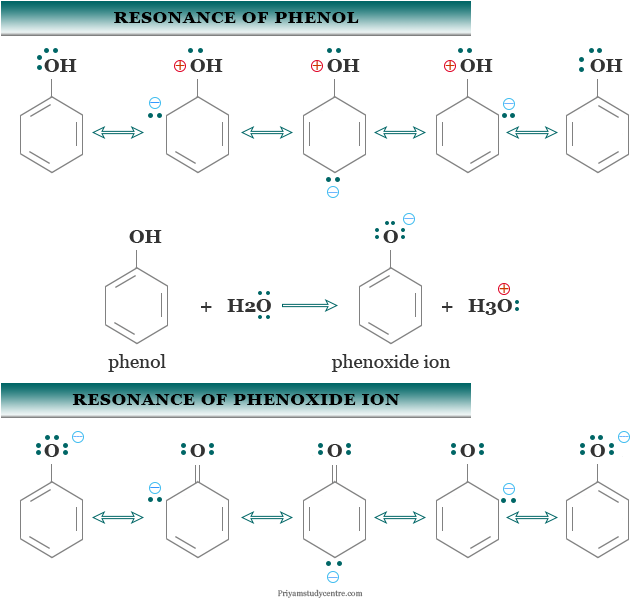
The acidity of C2H5OH is also explained by the stability of the phenoxide anion. The phenoxide ion has also resonance structures. It is more stabilized by resonance than the un-ionized C2H5OH molecule due to the spreading of the negative charge.
Solubility of Phenol in Water
The solubility of phenol in water can be explained by hydrogen bonding with water molecules. Like other alcohols, it can also form hydrogen bonds with water molecules.
The solubility of C6H5OH is much less than that of other alcohols due to the presence of the benzene ring. With the increasing hydroxyl group, the solubility of C6H5OH in water increases.
Chemical Reactions
Most of the reactions of phenol are connected with the benzene ring rather than the hydroxyl group.
- It gives a violet colour with ferric chloride.
- C6H5OH behaves as a weak acid and forms phenoxide salts with strong alkalies.
C6H5OH + NaOH → C6H5ONa + H2O
Alkali phenoxide reacts with alkali halide to form phenolic esters. - It forms phenyl esters when warmed with an acid chloride or acid anhydride.
C6H5OH + (CH3CO)2O → CH3COOC6H5 + CH3COOH - Many of the reactions of C6H5OH are carried out by electrophilic aromatic substitution in ortho and para positions. For example, the alkylation of C6H5OH forms p-derivative with a small amount of o-derivative.
- It can be hydrogenated in the presence of a nickel catalyst at 160 °C to form cyclohexanol.
C6H5OH + 3H2 → C6H11OH
Phenol Formaldehyde Resin
At low temperatures, in the presence of dilute acid or alkali phenol condensed with formalin solution which contains 40 percent aqueous formaldehyde to form ortho and para-hydroxy benzyl alcohol. It is the basis of the preparation of phenol-formaldehyde resin or Bakelite.
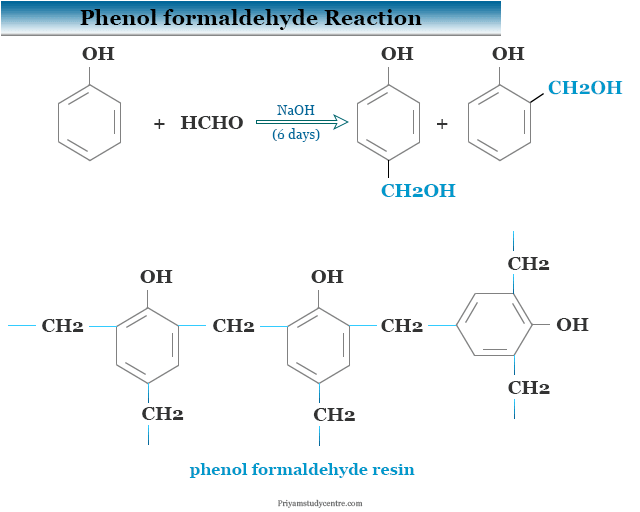
Production Process
It can be first extracted from coal tar. It is the oldest method for the extraction of phenol. However, such a type of extraction is now insufficient to give the required amount of phenol for the industry. Therefore, we prepared it synthetically.
The cumene process is the most important process for the production of C2H5OH. It is carried out by the oxidation of cumene to its hydroperoxide. It is decomposed by acid to form phenol and acetone. Both products have commercial importance.
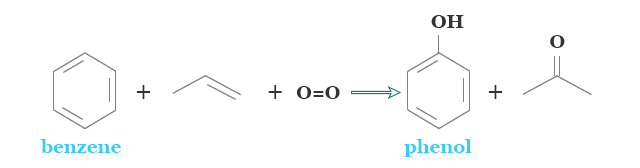
It is also prepared by oxidation of toluene by air in the presence of a chemical catalyst such as manganous or cupric salts.
C6H5CH3 → C6H5OH + CO2
It is produced by the steam distillation of benzene diazonium sulfate solution.
C6H5N2HSO4 + H2O → C6H5OH + N2 + H2SO4
It may be prepared by the fusion of sodium benzyl sulphonate with sodium hydroxide. It is an early commercial route for the production of C6H5OH.
C6H5SO3Na + 2NaOH → C6H5ONa + Na2SO3 + H2O
What is Phenol Used For?
- A major part of phenol can be used for the production of phenolic resins.
- It is the base material for the manufacture of nylon and other synthetic fibers.
- Phenolic resins can be prepared by the reactions of phenolic compounds with formaldehyde. The most famous example of phenolic resins is Bakelite.
- The phenol-chloroform extraction technique in molecular biology is used for the separation of nucleic acids such as DNA and RNA from proteins and lipids.
- It is also used for the production of different types of household products, antiseptics, and disinfectants.
- Phenol is the first antiseptic and disinfectant that we use in surgical instruments.
- Phenolic compounds such as salicylic acid, salol, aspirin, methyl salicylate, and phenacetin can be used for making different types of drugs in the pharmaceutical industry.
- Due to its antiseptic and disinfectant properties, C6H5OH is used for the production of different types of cosmetics like carbolic soap, sunscreens, hair colorings, skin lightening, or toners. Such types of use can be banned by the European Union and Canada due to their safety concerns.
- Phenol can be used for the production of explosive picric acid, indicator phenolphthalein, and solvent cyclohexanol.