Buffer Solution and pH
Buffer solution simply called buffer in analytical chemistry or biology is a solution whose pH remains virtually unchanged upon the addition of small amounts of strong acids or bases. A buffer solution may be made up of an acid and its conjugate base or a base and its conjugate acid. In the laboratory, buffer solutions are extensively used for the analysis of different types of precipitation which is carried out at specific pH ranges. In the industrial chemical process, various operations are pH-controlled. Therefore, we used buffer action to control such types of operations. If 1 cc of 0.01 (N) HCl is added to 1 liter of pure water, its pH changes from 7 to 5. But if the same amount of acid is added to 1 lit of buffer solution containing 0.1(M) acetic acid and 0.1(M) sodium acetate, the pH of the solution remains virtually the same.
Some of the common buffers that we used in the lab with their pH ranges are given below in the table,
| Buffer solution | pH range |
| Acetic acid and sodium acetate | 3.7 to 5.77 |
| Phthalic acid and potassium acid phthalate | 2.2 to 3.8 |
| Potassium acid phthalate and dipotassium phthalate | 4.0 to 6.2 |
| Na2HPO4 and NaH2PO4 | 5.9 to 8.0 |
| Boric acid and borax | 6.8 to 9.2 |
| Borax and sodium hydroxide | 9.2 to 11.0 |
Types of buffers
Buffer solutions consist of either a weak acid or weak base and its salts. Therefore, buffers are broadly divided into two types,
- Acid buffer
- Alkali buffer
Acid Buffers
Acid buffers are the mixture of a weak acid and its salt. A mixture of acetic acid and sodium acetate in a certain proportion is an example of an acid buffer solution.
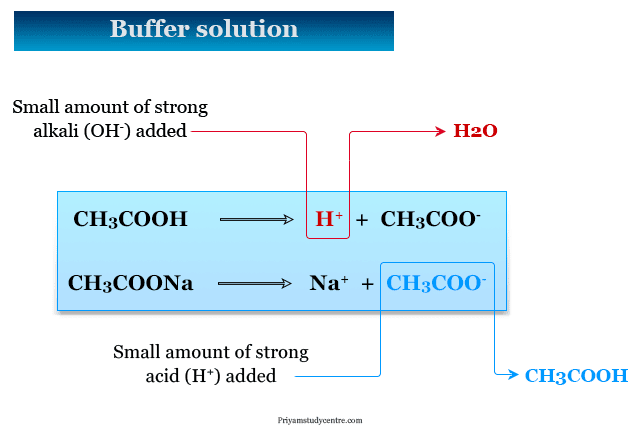
When we added a few drops of strong base sodium hydroxide to this acid buffer, the dissociated OH− ions could be consumed by weak acetic acid. Therefore, the pH of the buffer system remains unchanged.
Alkali Buffers
Alkali buffers are a mixture of a weak base and its salt. A mixture of ammonium hydroxide and ammonium chloride in a certain proportion is an example of an alkali buffer solution.
Buffer Action
The buffer action maintains a steady pH by adjusting the equilibrium between a weak acid or a weak base and its conjugate ions.
Let us consider the example of an acid buffer containing acetic acid and sodium acetate. When we added a small amount of strong acid to the above buffering system, the following reaction occurred,
H+ (added) + Ac− (buffer) → HAc
Therefore, H+ is removed from the solution.
When a small amount of strong alkali is added,
OH− (added) + HAc (buffer) → H2O + Ac−
Therefore, OH− is removed from the solution.
pH of Buffer Solution
To calculate the pH of a buffer solution made up of a weak acid and its conjugate salt, we can apply the Henderson equation,
pH = pKa + log[salt]/[acid]
If the concentration of the salt and the acid in the above relation is the same,
we have, pH = pKa
Therefore, to make a buffer solution of a particular pH, we used a weak acid whose pKa is close to the desired pH value. For example, a buffer of pH around 5. A mixture of acetic acid (pKa = 4.74) and ammonium acetate will be a good choice.
Similarly, for alkali buffering,
pH = pKb + log[salt]/[base]
Where pKb = dissociation constant of the base. The above equation is used to calculate the pH of the alkali buffer solutions.
Buffer Capacity
The efficiency of a buffer action to resist the change in pH on the addition of a small amount of strong acid or base is quantitatively expressed by the term called buffer capacity (β).
Buffer capacity can be defined as,
β = db/d(pH)
or, β = − da/d(pH)
Where db and da are the moles of base or acid added to one liter of the solution to change the pH of the buffer by unity. Therefore, buffer capacity is the number of moles of acid or base that change the pH of the buffer solution by one unit.
Uses of Buffer
Buffer solutions are used widely in analytical chemistry, biological laboratories, and different types of industrial operations to maintain the desired pH range.
- In analytical chemistry, many reactions in the laboratory are conducted within a narrow pH range. Buffer solutions are extensively used in these cases to maintain the desired pH range. Therefore, we used buffers in analytical chemistry for selective precipitation, solvent extraction, and titration of a particular compound.
- Buffers come to aid chemistry when studying reaction rates at constant pH. For example, the oxidation of iodide ions by hydrogen peroxide is a pH dependent reaction. Therefore, when pH is maintained, it becomes possible to study the effect of the concentration of I−.
- Buffing is required for pH determinations by colorimetric method, standardization of glass electrodes, and other EMF measurements.
- In the industrial chemical process, various operations are pH controlled. Therefore, we buffered such chemical processes to give the best results. Examples of such processes are electrodeposition of metals, tanning of leather, brewing of alcohols, manufacture of paper, etc.
- Buffering actions are very useful in the biological system. Enzyme catalyzed reactions are highly selective to pH. Therefore, the effective pH range of enzyme catalyzed reaction is maintained by the buffers.
- The pH of the human blood is controlled within the range of 7.3 to 7.5 by a buffer solution of carbonic acid and sodium carbonate.