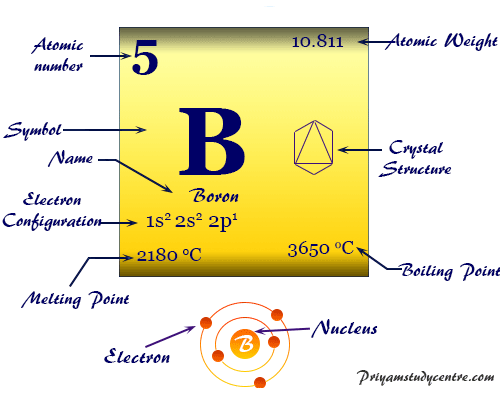
What is Boron?
Boron (symbol B), atomic number 5, is the first chemical element of Group 13 or Group IIIA in the periodic table. The different historical civilizations of the world uses borax (the compound of the element boron) as a flux to prepared glazes and hard glass. The compounds of boron such as borax and boric acid are also essential to plant growth and have a wide industrial application. The element boron has the smallest size and highest electronegativity among group 13 elements. The chemical properties like small size, high ionization energy, and moderate electronegativity (close to carbon and hydrogen) explain the formation of many exciting and unusual covalent bond or compounds of boron in chemistry. The element generally contains two isotopes, 10B (19.6 percent) and 11B (80.4 percent).
Who Discovered Boron?
The element itself was isolated in the nineteenth century by Davy, Gay Lussac, and Thenard by electrolysis of moist boric acid.
Moissan in 1892 prepared a rather pure specimen of boron (95 percent) by heating B2O3 with magnesium metal. The name was proposed by Devay to illustrate its source and similarities to carbon.
Properties of Boron
Pure boron is a high melting solid diamagnetic substance (melting point = 2180 °C). It is either a crystalline black or an amorphous brown solid. It is used as a semiconductor that conducts electricity at high temperatures like a metal.
| Boron |
||||
| Symbol | B | |||
| Discovery | Louis-Josef Gay-Lussac and Louis-Jacques Thénard in Paris, France, and Humphry Davy in 1808 | |||
| Name derived from | the Arabic name buraq, which is the name for borax | |||
| Allotropes | α-rhombohedral, β-rhombohedral, γ-rhombohedral and tetragonal | |||
| Common isotope | 11B | |||
| Crystal structure | Rhombohedral | |||
| Periodic properties | ||||
| Atomic number | 5 | |||
| Electron per shell | 2, 3 | |||
| Atomic weight | 10.81 | |||
| Electronic configuration | [He] 2s2 2p1 | |||
| Group | 13 | |||
| Period | 2 | |||
| Block | p-block | |||
| Physical properties | ||||
| State at 20 °C | Solid | |||
| Melting point | 2076 °C or 2349 K or 3769 °F | |||
| Boiling Point | 3927 °C or 4200 K or 7101 °F | |||
| Density | 2.08 g/cm3 | |||
| Chemical properties | ||||
| Atomic radius (non-bonded) | 1.92 Å | |||
| Covalent radius | 0.84 Å | |||
| Oxidation number or states | +3 | |||
| Ionization energy (kJ/mol) | 1st | 2nd | 3rd | |
| 800.64 | 2427.07 | 3659.75 | ||
| Electron affinity | 26.989 kJ mol−1 | |||
| Electronegativity | 2.04 (Pauling scale) | |||
| Molar heat capacity |
11.087 J mol−1 K−1 | |||
| CAS number | 7440-42-8 | |||
Boron on the Periodic Table
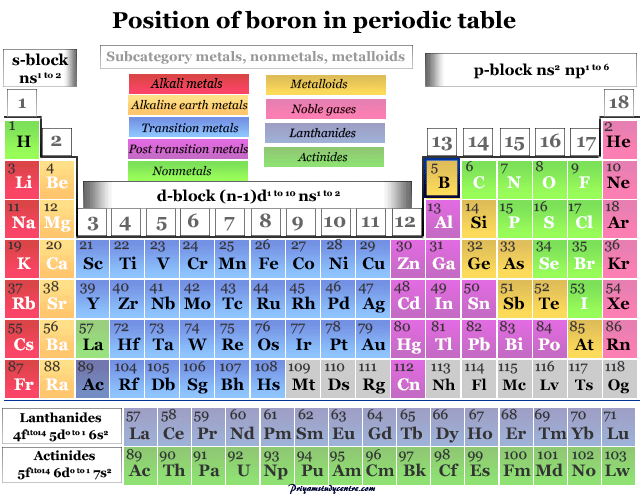
Boron in the Group 13 of the periodic table member occurs in a small amount due to its nucleus being disintegrated by natural bombardment reactions. On the other hand, another group 13 metal aluminum is very abundant and occupies the third position after oxygen and silicon.
Chemical Properties
- The crystalline elemental form of boron is chemically very inert and not affected by acids or oxidizing agents.
- Fused sodium hydroxide (NaOH) at 500 °C attacks it to form NaBO2 and hydrogen gas.
- The amorphous form is more reactive than the crystalline form. The amorphous elements of Group 13 (B, Al, Ga, In) burn in the air to form trioxides and nitrides.
- It dissolves in acid like nitric acid or sulfuric acid but is unreacted with hydrochloric acid.
- Only boron and aluminum combine directly with nitrogen when heated. These two elements form BN and AlN. But GaN and InN may be obtained indirectly by heating the metals with ammonia.
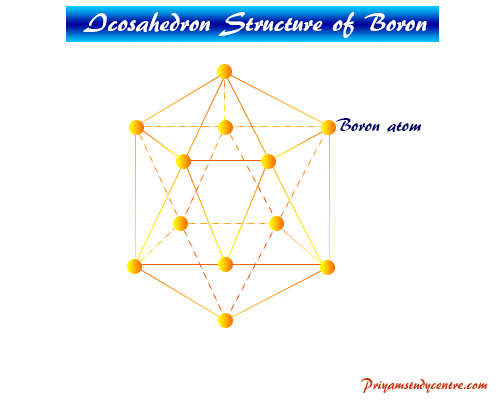
Allotropes
It exists in several allotropic forms which are structurally very complex, built up by B12 icosahedra. Different types of packing on icosahedra in unit crystal lattice form different types of structural formulas.
The simplest form is α-rhombohedral can be represented below picture.
Where Boron is Found?
Boron is found very low in crustal rocks (9 ppm) which is less than that of lithium (18 ppm) or lead (13.1 ppm). The principal minerals of boron are colemanite, kernite, and borax.
| Mineral | Composition |
| Colemanite | Ca2[B3O4(OH)3]2, 2H2O |
| Kernite | Na2[B4O5(OH)4], 2H2O |
| Borax | Na2[B4O5(OH)4], 8H2O |
The vast deposits of boron minerals like borate or borosilicates occurred in the regions of California, Turkey, Russia, and Argentina through former volcanic activities and water from hot springs. In India, Tibet, and Sri Lanka, borax occurs as a precipitate from hot springs.
Preparation Process
Crystalline Boron
The crystalline form of high purity element is now obtained by the reduction of volatile boron compounds (BCl3 or better BBr3) with hydrogen on heated titanium wire. However, the process can operate to form the element on a kilogram scale.
- Below 1000 °C, amorphous boron is obtained.
- Between 1000 to 1200 °C α and β-rhombohedral forms are obtained.
- Above 1200 °C tetragonal crystal is formed.
Amorphous Boron
In large quantities, the amorphous boron is obtained by the reduction of B2O3 with magnesium or other electropositive metals at high temperatures.
B2O3 + 3 Mg → 2 B + 3 MgO
The reaction is exothermic with the free energy at 298K = −515 kJ. The unreacted substances and MgO can be removed by washing the substance with dilute hydrochloric acid and sodium hydroxide solutions.
The 95 percent pure boron powdered is cheaply obtained by electrolytic reduction of KBF4 in molten potassium chloride (KCl) or potassium fluoride (KF) at 800 °C.
Uses of Boron Element
- The element boron and its compounds have been extensively uses in filaments, fiber composites, and reinforcement materials such as space shuttles, and aircraft.
- Boron carbides are used as abrasives for polishing or grinding. The carbides and metal borides are extensively used in neutron shielding and control rods for nuclear power plants.
- The isotope like 10B has a high absorption cross-section for high energy neutrons (104 to 106 eV). It is a more beneficial substance in B-10 neutron capture therapy of brain tumors.
- Metal borides (TiB2, ZrB2) have found many technical applications. Due to the physical and chemical properties like high melting point, hard nature, and chemically inertness of metal borides are used as a coating on turbine blades, rocket nozzles, and high-temperature reaction vessels.
- Boric acid, B2O3, and borax are used in agriculture and medicine. It is also used for making borosilicate glass, enamels, and fire retardants.
Chemical Compounds
The electron configuration of the boron is 1s2 2s2 2p1, with the two electrons in s-orbitals and one electron in the p-orbital. The fact suggests the monovalent state but it never exhibits a +1 oxidation number. It only shows the +3 state or number.
The chemistry of the first member of the boron family is naturally influenced by the small size and high ionization energy. Therefore, the compounds will be essentially covalent chemical bonding with the oxidation state III.
Depending upon the individual structure and chemical reactivity, the principal boron compounds may be classified into, hydrides and their derivatives (carboranes and polyhedral borane metal complexes), halides, or oxides compounds including polyborates, borosilicates, metal borides, boron nitride, and organoboron compounds.
Why boron does not form a B+3 ion?
The formation of B+3 ion does not form due to two main reasons. The ionization energy for the formation of a B+3 ion would be very large, and a small ion has highly polarizing properties. Due to the presence of three bond pairs, B+3 is a Lewis acid. Therefore, it accepts electron pairs from the Lewis base.
Interesting Facts About Boron
- The electron deficiency defines the unique characteristics of boron to form Pi-bonds with elements like chlorine, fluorine, etc. In BH3, BCl3, or BF3, the element attains noble gas configuration through dimerization of the molecule.
- Due to its small size, the boron atom can readily form a stable lattice with metal atoms (interstitial alloy). It also forms chain species with three-dimensional networks.