Lithium metal
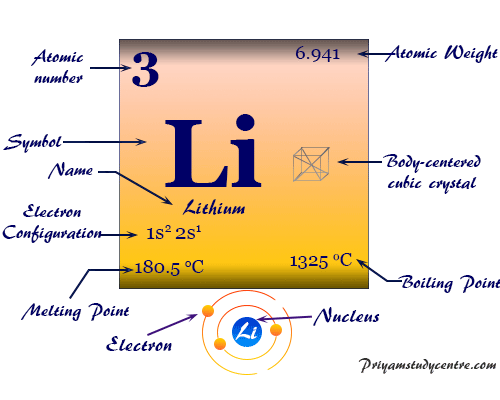
Lithium is the lightest solid chemical element or alkali metal of Group 1 or IA in the periodic table with the symbol Li and atomic number 3. It is widely used for the production of good alloys with aluminum and magnesium.
The systematic study of alkali metals (lithium, sodium, and potassium) in inorganic chemistry describes their physical and chemical properties. These can be readily understood by the outer electronic configuration.
The alkali metal, lithium is soft, low melting (melting point 180.5 °C), and silvery-white body-centered cubic crystal lattice at room temperature. But at low temperatures, it forms a hexagonal close pack structure.
Properties of lithium
The electron configuration of the lithium atom is 1s2 2s1. Therefore, the only 2s1 electron of the metal takes part in metallic bonding.
All the alkali metals give characteristic flame due to easy excitation by electromagnetic spectrum with the following colour,
- Lithium: crimson
- Sodium: yellow
- Potassium: violet
- Rubidium: red-violet
- Cesium: blue
This fact of alkali metals developed the analytical method for precise estimation by flame photometer.
The large difference between the first and second ionization energy of lithium suggests that the preferred oxidation number or state of metal will be +1. It is preferred to form chemical compounds by ionic chemical bonding in chemistry.
| Lithium | |||
| Symbol | Li | ||
| Discovery | Johan August Arfvedson in 1817 | ||
| Name derived from | The Greek word lithos means stone | ||
| Common isotope | 3Li7 | ||
| Oxidation state | +1 | ||
| CAS number | 7439-93-2 | ||
| Periodic properties | |||
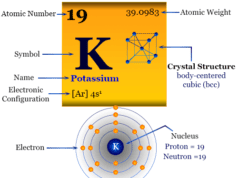
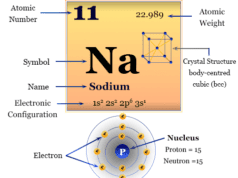
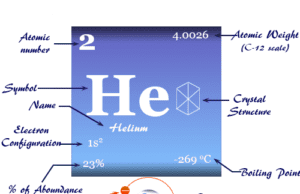
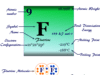
| Atomic number | 3 | ||
| Atomic weight | 6.941 | ||
| Electron per shell | 2, 1 | ||
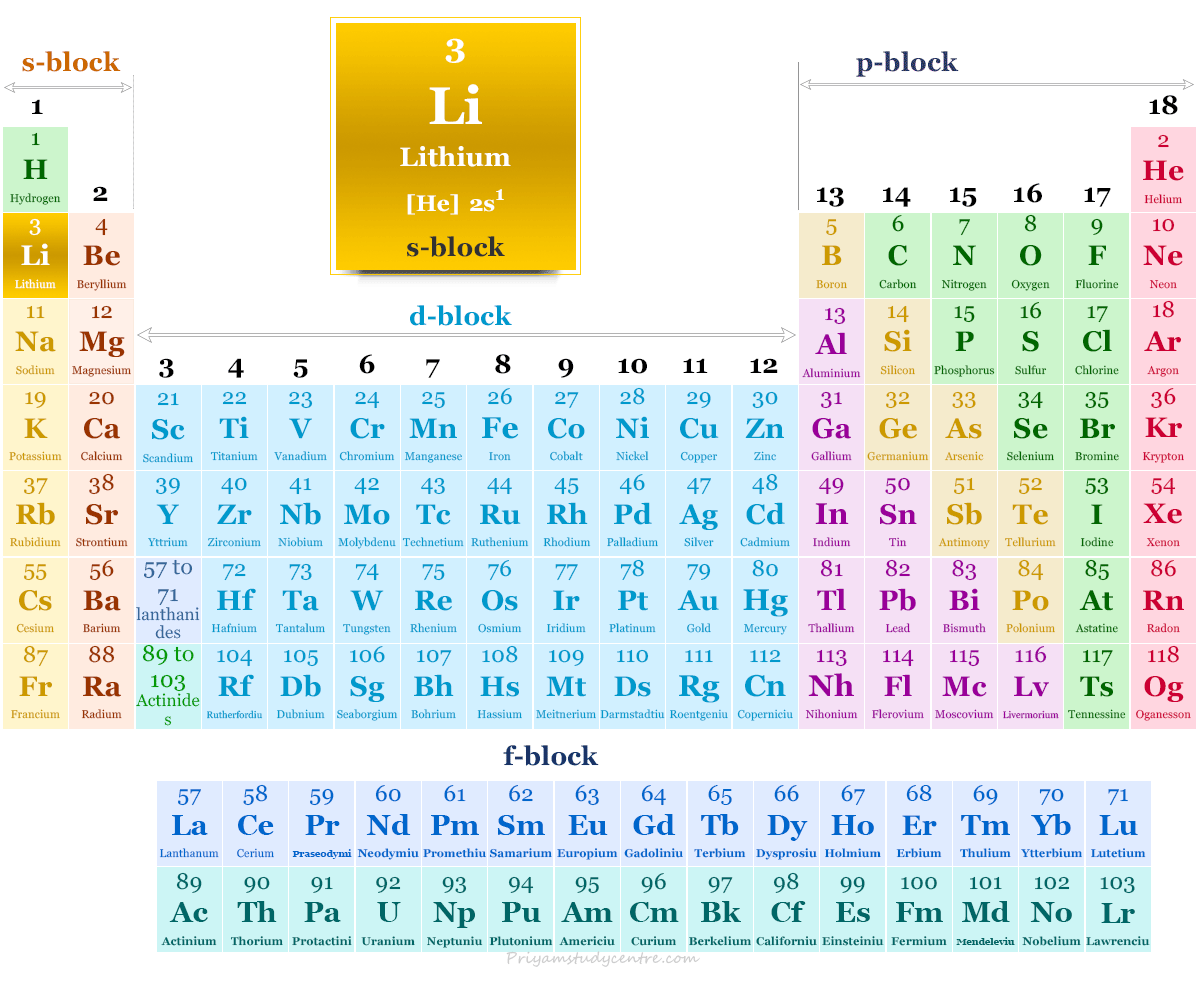
| Electronic configuration | [He] 2s1 | ||
| Group | 1 | ||
| Period | 2 | ||
| Block | s-block | ||
| Physical properties | |||
| State at 20 °C | Solid | ||
| Melting point | 180.50 °C, 356.90 °F, 453.65 K | ||
| Boiling point | 1342 °C, 2448°F, 1615 K | ||
| Density | 0.534 g cm−3 | ||
| Critical temperature | 3220 K | ||
| Molar heat capacity | 24.860 J mol−1 K−1 | ||
| Crystal structure | body-centered cubic (bcc) | ||
| Electrical resistivity | 92.8 nΩ m | ||
| Atomic properties | |||
| Atomic radius (non-bonded) | 1.82 Å | ||
| Covalent radius | 1.30 Å | ||
| Electron affinity | 119.16 kJ mol−1 | ||
| Electronegativity | 0.98 (Pauling scale) | ||
| Ionization energy (kJ mol−1) | 1st | 2nd | 3rd |
| 520.22 | 7298.15 | 11815.04 | |
Lithium in the periodic table
Lithium is placed in group 1 and period 2 of the periodic table. It is an s-block element that lies between hydrogen and sodium.
Who discovered lithium?
Lithium was discovered by Swedish chemist Johan August Arfvedson in 1817 in the mineral petalite. The name was chosen from Greek later lithos means stone. It occurs in crustal rocks (18 ppm) comparable to gallium (18 ppm) and niobium (20 ppm) in the earth’s environment.
Where is lithium found?
Aluminum silicate is the main mineral. It occurs in two forms,
- Spodumene, LiAl(SiO3)3 about 2.5 to 3 percent.
- Sepidolite, LiF, LiOH, Al2(SiO3)3 about 3.8 to 5.6 percent.
It is found mainly in the United States, Canada, Brazil, Argentina, the Soviet Union, Spain, and Congo.
The very low terrestrial or cosmic abundance of lithium compared to other alkali metals describe by its small nuclear charge and low potential barrier. It may facilitate nuclear reaction to heavier elements like beryllium or boron.
It also occurs in many springs and some radioactive decay minerals like carnallite, ashes of planets, in taboo, milk, and blood of living animals.
Searles Lake in California is an important source of nearly dry and alkaline (pH scale = 9.5) lithium metal.
In the 19th century, the United States was the largest producer of chemical elements and compounds like Li2CO3. In this century, Australia, Chile, and Portugal are the largest commercial suppliers of the metal or its compound.
Extraction process
The alkali metals beings most electropositive and have never been found in nature in elementary states. Due to electropositivity, alkali metals readily react with water and cannot be produced by electrolysis. Therefore, fused chloride and hydroxide are used for the preparation of lithium and sodium.
The major commercial form of the metal Li2CO3, is produced from ores or brines by different processes and uses largely in the chemical industry. About 1000 tonnes of lithium metal and several thousands of salts are prepared annually for various purposes of use.
Chemical reactivity
The large difference between the first and second ionization energy of lithium suggests that the preferred oxidation state of the metal is +1 oxidation. The major chemistry of metal is described by the Li+ ion.
In solution, the Li+ ion stabilizes by high solvation energy. The reactivity of the alkali family increases from Li to Cs, except for nitrogen molecules.
Chemical reactions
At 25 °C, water reacts with Li very slowly, sodium reacts vigorously, and potassium, rubidium, or cesium reacts with an explosion.
It is burnt in air or oxygen to form Li2O and a trace amount of Li2O2 but it reacts with nitrogen slowly at room temperature and rapidly to give ruby red crystalline solid Li3N.
The poor reactivity of the element is also illustrated by the fact that lithium cannot replace weekly acidic hydrogen from phenyl-acetylene but other alkali metals liberate hydrogen from this chemical compound.
Properties of lithium compounds
The properties or behavior of lithium and its compounds differ significantly from other alkali metals. The properties of Li resemble magnesium due to the diagonal relationship in the periodic table.
Many simple salts of metal are normally hydrated and anhydrous salts are hygroscopic in nature (LiCl, LiBr, LiI, etc). The structure of LiClO4, 3H2O, and Mg(ClO4)2, 6H2O are similar in nature, both contain octahedral groups.
The electrode potential for the redox reaction of lithium has the lowest value due to the highest ionization energy and small size of the atom.
What is lithium used for?
- Lithium forms low density and good alloy with aluminum and magnesium like LA-141 (Li = 14 percent, aluminum 1 percent, and magnesium 85 percent). This alloy is used for aircraft construction.
- Lithium stearate (LiOH + tallow) is used as a thinner or gelling agent for transforming oils into lubricating greases which are highly water-resistant materials.
- A compound like lithium carbonate is extensively used in cells for the extraction of aluminum by the larger flowing of current or reducing the production cost.
- It is used as a flux in making porcelain, enamel, and specially toughened glass
- Lithium is used in medicine for the treatment of manic-depressive psychoses.
- Lithium hydroxide is used for the absorption of carbon dioxide in space capsules and submarines.
- The elemental hydride uses to generate hydrogen for military or metrological requirements.
- Several organolithium compounds are widely used in the organic synthesis of hydrocarbons like alkanes or paraffin and alkenes or olefins.
- In storage batteries or cells, Li-Si and Li-Al alloy used as an anode in molten lithium chloride or potassium chloride are under trial to prepare electric current.
- Lithium may be used to prepare tritium (isotopes of hydrogen, which is a promoting fuel in the nuclear power generation process by the nuclear fusion reaction.