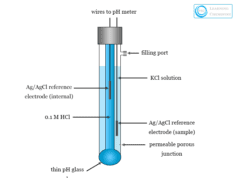
Ion Selective Electrode Principle
Ion selective electrode (ISE) is an electrochemical sensor that works based on the principle of a galvanic cell. It converts the activity or concentration of a specific ion present in a solution into electrical potential. It is very useful in environmental chemistry for the analysis of dissolved carbon dioxide from water samples.
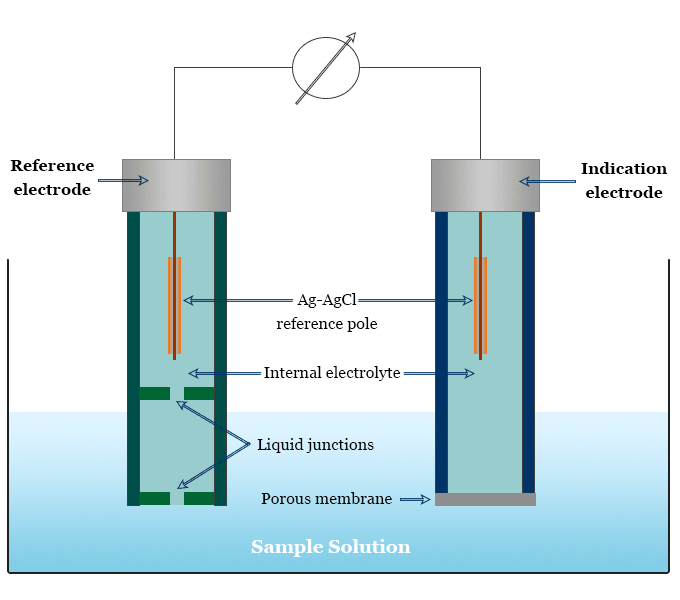
A typical assembly of an ion selective electrode is given above the picture.
Ion selective electrode can be used for the potentiometric determination of several anions (fluoride, chloride, bromide, iodide ion) and cations (sodium, potassium, calcium ion). The electrode can also be used for endpoint detection in potentiometric titration.
In biochemistry, ion selective electrodes are used for the determination of enzymes and substances which interact with enzymes.
How do Ion Selective Electrodes Work?
Potentiometric ion-selective electrode (ISE) is one of the most important groups of chemical sensors. The working process of potentiometric ion selective electrodes is based on the principle of a galvanic cell. It contains a reference electrode, ion-selective membrane, and voltmeter.
The transport of ions from high concentration to low concentration through the selective membrane creates a potential difference. It can be measured with respect to a standard reference electrode having a constant electrode potential. Under equilibrium conditions, the measured potential (E) can be expressed by the Nernst equation.
Ecell = E0 + (0.059/n) log aMn+ at 250 C
Where n is the charge of the analyte ion, aMn+ is the activity of the sample solution, and E0 is asymmetric potential. It is developed across the membrane due to internal strain. It is the characteristic constant of each ion selective electrode.
To determine the value of E0, the electrode is dipped into a solution of known pH value and coupled with a secondary reference electrode such as a calomel electrode.
Ion Selective Electrode Types
The essential part of an ISE is the ion-selective membrane which is commonly placed between the sample and inner solutions that contain an analyte ion. The membrane used in ISE may be a glass, a crystalline solid, or a liquid. Ion selective electrodes are mainly four types according to the nature of membrane material. These are,
- Glass electrodes
- Crystalline electrodes
- Ion exchange electrodes
- Enzyme electrodes
Glass Electrodes
A glass electrode is a type of ISE where a thin bulb shape glass membrane is used as a sensing material. It is sensitive to hydrogen ions in the solution. When a thin glass membrane separates two electrolytes, a potential is developed across the membrane.
A carbon dioxide sensitive electrode is a pH-sensitive glass electrode. It is very useful in environmental chemistry for the analysis of dissolved carbon dioxide in water samples.
The glass electrode has good selectivity for silver (Ag+), sodium (Na+), and hydrogen (H+) ions. While chalcogenide glass has good selectivity for double-charged metal ions like cadmium (Cd2+) and lead (Pb2+) ions.
Crystalline Electrodes
Solid-state (crystalline) membrane electrodes contain an insoluble inorganic salt. Crystalline electrodes have good selectivity. The ions which can introduce themselves into the crystal structure can interfere with the electrode response. An ion-exchange process leads to the formation of potential at the membrane of crystalline electrodes.
Mono- or polycrystalline of a single substance such as AgCl / Ag2S can be used to determine the activity or concentration of chloride ions.
Single crystal LaF3 is widely used to determine the activity of fluoride ions. To improve the conductivity, the crystal is usually doped with europium. It was described by Martin and Frant in the year of 1960.
Ion Exchange Electrodes
Ion exchange electrodes are based on the membrane which is electrically conductive. An ion-exchange membrane is a type of organic polymer or ion exchange resin that transports specific dissolved ions from the solution and blocks other ions or neutral molecules.
Important examples of ion-exchange membranes are proton-exchange membranes and anion exchange membranes.
- Proton-exchange membranes are used to transport H+ ions.
- Anion exchange membranes are used in certain alkaline fuel cells to transport OH− ions from dissolved solution.
Potassium-selective electrode is a great example of liquid membrane ion exchange electrode where valinomycin uses as an exchanger. It has high selectivity against other alkaline metal cations and ammonium cations. The sensing membrane is based on a polyvinyl chloride matrix. It can be used for several months in clinical analysis.
Enzyme Electrodes
Enzyme electrodes are based on the principles where enzymes can interact with substrates in biological processes. It was first developed by Clark and Lyons.
An amygdalin-sensitive electrode can be made by retaining β-glucosides in a gel layer coupled to a cyanide-sensitive membrane electrode. Glucose and urea can be measured by enzyme electrodes. It is beneficial for diabetic patients.
In clinical application, enzymes play a critical role in the metabolic activities of all living organisms. Therefore, enzyme electrodes are broadly applied in biotechnology and clinical analysis.
Application of Ion Selective Electrode
The application of ion selective electrodes (ISEs) has been well-established in many fields such as clinical, biological, water, air, oceanographic, pharmaceutical research, and analytical determinations.
The application of fluoride, chloride, ammonium, sodium, potassium, and calcium ion selective electrodes are given below,
Fluoride Ion Selective Electrode
A fluoride ISE is a type of ion selective electrode which used to measure the concentration of the fluoride ion in aqueous solutions quickly, simply, accurately, and economically. It is a useful test procedure for the measurement of fluoride in drinking water, bone, cement, fish protein, glass, phosphate-mineral, and toothpaste.
Chloride Ion Selective Electrode
The chloride ion selective electrode is used for a rapid, simple, and reliable analysis of chloride ions in biological materials, food products, soils, and wastewater. It is used mainly for the determination of the salinity of water and chloride ions from blood serum, urine, fish, plant tissues, milk, beef extract, nutrient broth, and orange, tomato, and grapefruit juices.
Ammonium Ion Selective Electrode
The ammonium ion-selective electrode can be used to replace the distillation and titration procedure. It can be used to measure the concentration of ammonium (NH4+) ion in aqueous samples. After the conversion of nitrogen to an ammonium ion, the ammonia concentration is determined with the ammonium electrode.
Ammonium ISE is used mainly to measure levels of ammonium ions present in fertilizers.
Sodium Ion Selective Electrode
The sodium ion-selective electrode is designed to detect sodium ions (Na+) from aqueous solutions. It contains a solid-state PVC polymer matrix membrane. It is suitable for various laboratory applications.
Detection of sodium from blood, food, and soil is very important for modern people. Sodium is one of the essential constituents in the blood and extracellular fluid.
Sodium with potassium maintains various biological functions such as osmotic pressures and moisture inside and outside the cell. They also operate functions of nerves, heart, muscles, etc.
Excessive sodium level in the human body causes high blood pressure or hypertension. Therefore, controlling sodium levels in our body is very important for modern people.
Potassium Ion Selective Electrode
A potassium ISE is an example ion selective electrode used for measurements of potassium ion concentration in an aqueous solution. Highly selective potassium ion exchangers and robust polymeric membranes are used widely in biochemical and biophysical research.
In advanced analytical chemistry, these types of membranes can be used to measure the potassium activity in plasma or whole blood.
Calcium Ion Selective Electrode
A calcium ion selective electrode is a membrane-based electrode which used to measure Ca2+ ion concentration or activity in an aqueous solution. It is very useful in determining the hardness of the water.
Calcium ISE is also used to determine calcium ions in beer, soil, feedstuffs, flour, minerals, milk, seawater, serum and biological fluids, sugar, pulping liquor, and wine.
A recent development is the use of liquid ion selective membranes in ion selective electrodes. These liquid ion exchange membranes are non-volatile, water-immiscible chelates or coordination complexes.
Fluoride (F−), chloride (Cl−), bromide (Br−), iodide (I−), cadmium (Cd2+), copper (Cu2+), lead (Pb2+), nitrate (NO3−), potassium (K+), silver (Ag+), sodium (Na+), ammonia (NH3), and several enzymes ion selective electrode are most common, reliable and commercially available electrodes used in the various analysis.