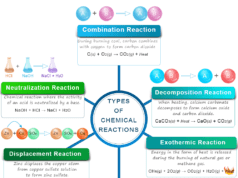
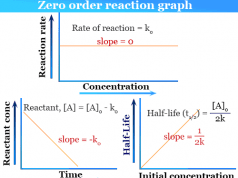
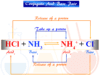
Catalyst in Chemistry
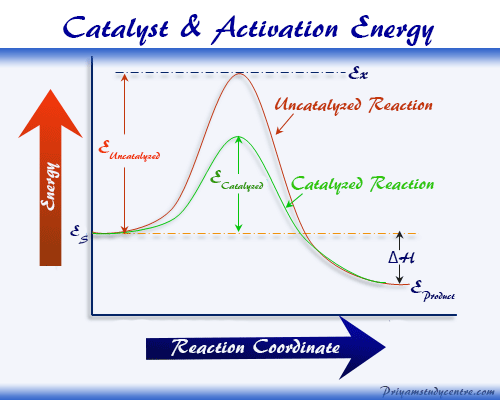
Chemical catalyst or simply catalyst is a foreign substance that increases the speed or rate of the reaction by lowering activation energy without change at the end of the chemical or biological process. A catalyst in chemistry can be simply defined as a substance that enhances the speed or velocity of the chemical reaction itself remaining unaltered in mass, activity, and chemical composition. Catalysts play an important role in many chemical and real-life biological processes and work to find the alternate path where activation energy decreases but kinetics reaction rate increases. Chemical catalysts are mainly two types, homogeneous catalyst and heterogeneous catalyst. The phenomenon where catalysts show their activity is defined as the catalysis reaction in chemistry. For example, hydrogen ion acts as a positive catalyst in the hydrolysis of ester or sugar without affecting chemical equilibrium.
Negative Catalyst
Sometimes the foreign substance slows down the speed of the chemical synthesis process or even stops the reaction is called a negative catalyst.
However, such a common definition does not fully satisfy negative catalysts because negative catalysts sometimes change the chemical process permanently. Therefore, such substances in chemistry are properly called inhibitors. For example, sulfuric acid acts as an inhibitor in the decomposition of hydrogen peroxide.
Characteristics of Catalyst
The catalyst uses in the catalysis reaction has some common and definite characteristics in chemistry or chemical science.
The most important characteristics of the catalyst remain unchanged in the molecular mass, density, concentration, and chemical formula of constitute elements at the end of the reaction. However, the physical state of chemicals like particle size or color of the chemical catalyst may be altered.
For example, manganese dioxide uses for the decomposition of potassium chlorate becomes a finely divided powder after the chemical reaction is completed.
A catalyst can increase the rate or speed of chemical reactions. It can not start a reaction but only increases or decreases the speed of the reaction.
The catalyst does not affect the final state of chemical equilibrium. This means the catalyst itself is not consumed during the chemical reaction. It regenerates at the end of the reaction.
For example, 1.7 g of platinum produces 1.8 cc of oxygen per minute from the decomposition of hydrogen peroxide. But the platinum remains active even production of 10 liters of oxygen in a catalysis reaction.
Effect of Catalyst on Chemical Equilibrium
The catalyst does not affect the final state of equilibrium derived from Van’t Hoff equation,
ΔG0 = − RT lnk
where k = equilibrium constant
Since the catalysts do not contribute any energy to the system to increase the free energy but increase the entropy of the system. The thermodynamics definition or equation of free energy shows the fact that catalysts do not change the free energy and equilibrium constant of the chemical reaction.
The equilibrium constant,
k = k1/k2
where k1 and k2 = rate of forward and backward reaction
If the catalyst increases the rate of the forward process or k1 increases, to keep k constant, k2 also increases to the same extent.
Therefore, catalysts enhance the rate of both types of forward and backward reactions, which helps to attain equilibrium more quickly but it does not affect the equilibrium according to the Le Chatelier principle.
Examples of Catalysts
From the definition and example, catalysts play a specific role in a specific catalytic reaction in science. Therefore, we can not use the same common catalysts for every chemical process.
For example, manganese dioxide can catalyze the decomposition of potassium chlorate but not potassium nitrate. The change of catalysts defines the nature of the chemical product of the reaction in chemistry.
For example, carbon monoxide and hydrogen in the presence of nickel catalyst produce methane gas but in the presence of zinc oxide produce methyl alcohol molecules.
| Chemical reactions | chemical catalyst |
| CO + H2 → CH4 + H2O | presence of Ni |
| CO + H2 → CH3OH | presence of ZnO |
| HCOOH → H2 + CO2 | presence of Cu or ZnO |
| HCOOH → H2O + CO | presence of Al2O3 |
An optimum temperature at which the efficiency or role of natural catalysts like enzymes is most marked in chemical science and biology.
In biological catalysis reactions, the catalytic action of the enzyme increases with the rise of specific heat or temperature. However, the efficiency falls after a certain temperature due to the coagulation of the enzyme.
Different Types of Catalyst in Chemistry
In real life, the most common chemical catalysts and catalyzed reactions are classified into two types,
- Homogeneous catalyst
- Heterogeneous catalyst
Homogeneous Catalyst
In inhomogeneous types of catalysis reactions, the catalyst forms a single phase with the reactants. Therefore, many homogeneous catalyzed reactions have been studied in the gas phase or liquid phase.
Examples of Homogeneous Catalysts
The reaction rate in a homogeneous catalysis reaction is invariably proportional to the concentration of the catalysts.
The most common examples of such catalysis chemical reactions in the gas phase are,
- Oxidation of sulfur dioxide to sulfur trioxide catalyzed by nitrogen dioxide.
- Decomposition of nitrous oxide to nitrogen and oxygen catalyzed by NO2.
- Decomposition of ethers or acetaldehyde by iodine catalyst.
In heterogeneous catalysis, the chemical catalysts and reagents form separate phases, and often the surface or interface is responsible for the catalytic properties.
Heterogeneous Catalyst
Heterogeneous catalysis reactions mean, a solid serves as a catalyst and the reactants are gaseous or liquid phases. One or more of these reactants are absorbed on the surface of the solid. Therefore, the surface provides an alternative path for lowering activation energy or accelerating the reaction rate.
In most reactions, the heat of adsorption increases the activation energy of the reactant, and the equilibrium is reached easily. The reaction of the solid surface catalysts consists of four consecutive chemical steps,
- Diffusion of gases to the surface
- Adsorption of the gases on the surface
- Reactions on the surface
- Desorption or diffusion of products from the surface to the bulk.
The diffusion and desorption process is very rapid and does not play an important role in heterogeneous catalysis reactions.
Heterogeneous Catalysts Examples
The catalytic function on the surfaces of catalysts is specific in nature and different catalytic elements are effective for the different types of catalytic reactions.
| Homogeneous reaction | Catalyst examples |
| hydrogenation/ dehydrogenation | Nickel, copper, ZnO, Cr2O3 |
| dehydration/hydration | Al2O3 |
| cracking of petroleum | SiO2-Al2O3 gel |
| synthesis of ammonia | iron |
| polymerization | Al(Et)3, TiCl4 |
Transition metal surfaces like nickel or copper are a very good list of heterogeneous surfaces for the hydrogenation of alkenes or olefin. Alumina is also a suitable catalyst for the synthesis of hydrocarbon from a secondary alcohol.
These are due to the fact that hydrogen is strongly absorbed by the nickel or copper catalysts surface but water molecules are strongly absorbed by the alumina catalyst surface.