Platinum Metal
Platinum is a chemical element or silvery white, lustrous, malleable, high density metal of group-10 of the periodic table with atomic number 78 and symbol Pt. Chemically, platinum metal is inert to attack by mineral acids, air, or water under ordinary conditions. It is more ductile than gold, silver, or copper but less malleable than gold. The elements namely ruthenium, rhodium, palladium, osmium, iridium, and platinum are called platinum metals. All these elements are rare in the earth’s crust. Platinum metals are found together with other noble metals like copper, silver, and gold. It has many similarities with group member Palladium. +2 and +4 oxidation number or states are the most common for both metals.
History of Platinum
Platinum was found in the Tomb of queen Shapenapit in the 7th century. In 1748, the Spanish expeditions Antonio de Ulloa maintained certain minerals which contained metal with a very high melting point. It was called Platina (little silver in Spanish)
A subsequent investigation by H Scheffer, Von Sickingen, and P Charbonneau developed the isolation process and name of platinum metal.
Properties of Platinum
Generally, platinum metals have a high melting point and density.
The gradual entry of shielding electron or (n−1) electrons inner core is responsible for slowly decreasing melting point and density along with horizontal series from Re to Pt.
| Platinum Metal | Density (gm/cm3) | Boiling point (°C) |
| Ru | 12.45 | 2334 |
| Rh | 12.41 | 1964 |
| Pd | 12.023 | 1554 |
| Os | 22.59 | 3033 |
| Ir | 22.56 | 2446 |
| Pt | 21.45 | 1768 |
The electronic configuration and some physical and chemical properties of platinum are given below in the table,
| Properties | |
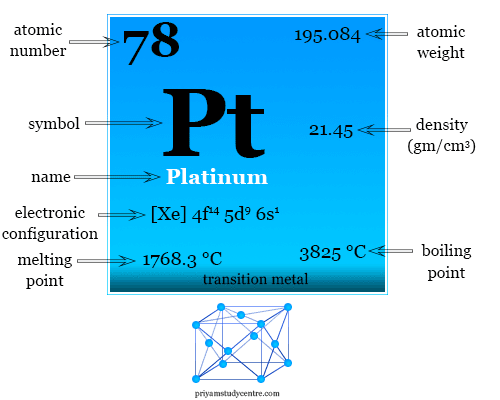
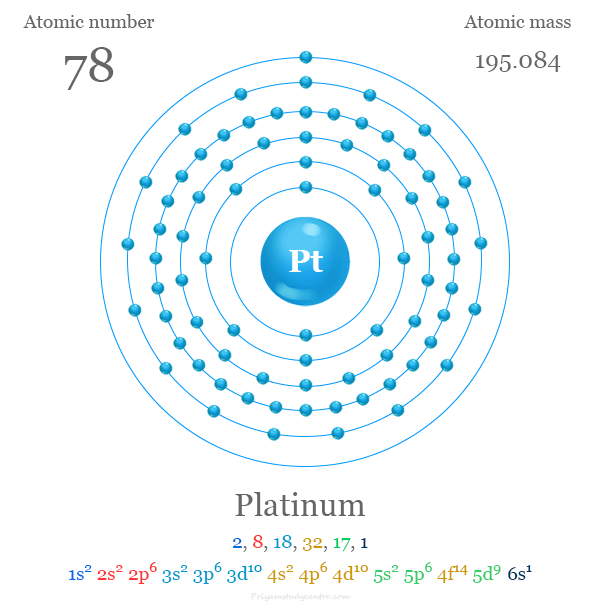
| Atomic number | 78 |
| Electronic configuration | [Xe] 4f14 5d9 6s1 |
| Atomic weight | 195.084 |
| Melting point | 1768.3 °C |
| Boiling point | 3825 °C |
| Density | 21.45 g/cm3 |
| Molar heat capacity | 25.86 J mol−1 K−1 |
| Electrical resistivity | 105 nΩ·m |
| Crystal structure | face-centered cubic (fcc) crystal lattice |
| Group | 10 |
| Period | 6 |
| Block | d-block |
| Chemical properties | |
| Oxidation number | +2, +4 |
| Electronegativity | 2.28 (Pauling scale) |
| Ionization energy | 1st: 870 kJ/mol 2nd: 1791 kJ/mol |
From group-10 elements like Ni, Pd, and Pt, the ionization energy increases with the increasing atomic number. Due to lanthanide construction, the atomic radius of Pd and Pt are the same.
Platinum in Periodic Table
It is placed in group 10 of the periodic table with d-block elements or transition metals. The valence shell electronic configuration of platinum is [Xe] 4f14 5d9 6s1.
Where is Platinum Found?
The chief sources of platinum are South Africa, Canada, Russia, Brazil, Columbia, etc. It is rare in the earth’s crust and found about 0.01 ppm. The metal is found together mostly with sulfide and arsenides of Cu, Ag, and Au.
Braggite (Pd, Pt, Ni)S, sperrylite (PtAs2) and cooperate (PtS) are the most common minerals of platinum metal. A native metal is also available in the earth’s environment.
Production Process
Platinum metals are mostly obtained from copper or nickel ores. In the production of copper or nickel, the anode slime contains platinum metals together with Ag and Au. Pt is collected from the anode slime by the treatment of aqua regia. The solution contains soluble chloro complexes like HAuCl4, H2PdCl4, and H2PtCl6.
- From the solution, gold is precipitated by FeSO4.
- The solution containing PdCl4−2 and PtCl6−2 is treated with NH4Cl to precipitate (NH4)2PtCl6. It is ignited to obtain impure spongy platinum metal.
- It is again dissolved in aqua regia and the solution is evaporated with sodium chloride. From the solution, Na2PtCl6 is obtained. If it contains Rh or Ir, remove it by adding NaBrO3.
- (NH4)2PtCl6 is now reprecipitated by adding NH4Cl and ignited to produce platinum metal.
Uses of Platinum Metal
- It is largely used as a chemical catalyst in different types of chemical reactions.
- It is used in reforming hydrocarbons, oxidation of ammonia to NO, and oxidation of sulfur dioxide (SO2) to sulfur trioxide (SO3).
- Pt is also used for making laboratory apparatus like a crucible, boat, etc.
- It is used for making electrical apparatus and resistance wire.
- Platinum electrodes are used for the measurement of conductance and electrochemical cells.
- At present, it is largely used in car exhaust converters to reduce the proportion of pollution.
- Cis-Pt(NH3)2Cl2 (cisplatin) is an effective antitumor drug used in cancer chemotherapy. A very high rate of success has been reported by the use of Cis-Pt(NH3)2Cl2 in the treatment of solid tumors, particularly the genito-urinary treatment.
- A considerable amount of Pt metal is used for making jewelry.
Chemical Compounds
+4 and +2 oxidation states are the most common oxidation states of Pt. Only a few chemical compounds are formed in +6 and +5 states. Some common chemical compounds of platinum metal in different oxidation states are discussed below,
Platinum Hexafluoride
The hexafluoride (PtF6) is the representative of platinum metal in the +6 oxidation state. It is a dark red volatile unstable solid.
PtF6 is a highly reactive and corrosive agent which reacts with glass in dry conditions. Therefore, to handle PtF6, we used an apparatus made of Monel metal. PtF6 is the most powerful oxidizing agent which oxidizes oxygen and xenon to form O2+PtF6− and Xe(PtF6)n.
Platinum (IV) Chloride
It forms all four tetrahalides. Yellow brown PtF4 is obtained by fluorination of platinum metal. Other tetrahalides are obtained by direct reaction of Pt with chlorine, bromine, or iodine.
The tetrachloride (PtCl4) is commonly prepared by heating H2PtCl6 in Cl2 at 300 °C. It is also prepared by reacting the Pt with SO2Cl2. The red-brown crystalline solid, PtCl4 dissolved in water to give ions having the composition [PtCl4(OH)2]−2.
Pt (II) Chloride
Pt (II) chloride is obtained by thermal decomposition of PtCl4. PtCl2 forms metal cluster compounds with the formula Pt6Cl12. In the Pt6Cl12 structure, the six platinum atoms occupy the six-point of the octahedron and the twelve edges of the octahedron contain twelve briding chlorine atoms.
Chloroplatinic Acid
Chloroplatinic acid or dihydrogen hexachloroplatinate (IV) is an important compound of platinum metal having the molecular formula H2[PtCl6]. It is obtained by dissolving Pt in aqua regia. Evaporating the solution with hydrochloric acid for the removal of nitric acid.
2 HNO3 + 8 HCl + Pt → H2[PtCl6] + 2 NOCl + 4 H2O
Chloroplatinic acid is a strong acid, it forms alkali or ammonium salts when reacting with alkali or ammonium hydroxide.
Platinum (IV) Complexes
Pt(IV) forms a large number of octahedral complexes that are stable and inert towards substitution. Many of these are readily obtained by adding ligands in Pt (II) complexes.
PtIIL4 + Br2 → trans-PtL4Br2
Whole series of Pt (IV) complexes ranging from [Pt(am)6]+4 (am = ammonia or a variety of amines) to [PtX6]−2, where, X = halogen, OH, CNS, NO2, etc.
The most common examples are,
- [Pt(NH3)6]Cl4
- [Pt(NH3)5Cl]Cl3
- [Pt(NH3)4Cl2]Cl2
- [Pt(NH3)3Cl3]Cl3
- K2[PtCl6]
Platinum (II) Complexes
Shaking a solution of K2PtCl4 in dilute HCl and ethylene (C2H4) gives the complex K[PtCl3(C2H4)] or Zeise’s salts. Cis and trans [Pt(NH3)2Cl2] are the most common examples of Pt (II) complexes.
Magnus’s green salt is another Pt (II) complex having the formula of [Pt(NH3)4][PtCl4]. This platinum salt is prepared by adding pink complex [PtCl4]−2 to colorless [Pt(NH3)4]+2.