Bond Energy and Dissociation Energy
Bond energy or bond enthalpy is the average value of the dissociation energies of a given bond in a series of different dissociating species. However, bond dissociation energy is the energy absorbed per mole when a particular kind of chemical bond is broken in a gaseous state of matter. The bond enthalpy and bond dissociation energy depend on the nature of atoms united by a bond and the nature of the molecule. For a diatomic molecule, these two energies are equal values. The calculated bond dissociation energy for breaking the oxygen hydrogen bond in the water molecule will be 120 kcal but when the second hydrogen atom is separated from the residual OH-group the dissociation energy = 101 kcal. The bond dissociation energy calculation formula provides the average value of bond energy of a given bond in a series of different dissociating species.
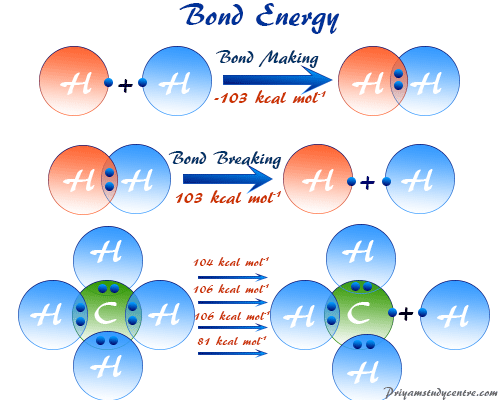
The bond dissociation energies for two bonds in a water molecule are 120 and 101 kcal. Therefore, the average value of the bond enthalpy = 110.5 kcal. The dissociation energy is generally equal to the bond enthalpy for a diatomic molecule. Therefore, bond energy is the average dissociating energy of a given bond in a whole molecule. The dissociation energy of the carbon-hydrogen bond in methane and benzene is different because the residual portion of these two molecules is different.
Bond Energy of Methane
A methane molecule is formed by the overlap of four sp3 hybridized orbitals of carbon and four 1s orbitals of the hydrogen atom. Therefore, all carbon-hydrogen bonds are equivalent. But dissociation energy required to break the first bond is not the same as the second bond.
For the calculation of bond energy, we can generally take the average values of all four bond dissociations. Therefore, the bond energy for methane molecule = 397/4 = 99 kcal.
The dissociation energy of some chemical bonds is given below in the table,
| Molecule | Chemical Bond | Dissociation Energy | |
| kJ/mol | Kcal/mol | ||
| Methane | CH3 – H | 426.8 | 102 |
| Ethane | CH3CH2 – H | 405.8 | 97 |
| Propane | Me2CH – H | 393.3 | 94 |
| t-butane | Me3C – H | 374.5 | 89.5 |
| Ethane | H3C – CH3 | 341.1 | 83 |
| Ethylene | H2C = CH2 | 710 | 174 |
| Acetylene | HC ≡ CH | 960 | 230 |
| Oxygen | O – O | 498 | 119 |
| Nitrogen | N ≡ N | 945 | 226 |
Ionic Resonance Energy
The greater the polarity of a bond greater be bond dissociation energies. This extra energy appearing in A – B molecules arises by electrostatic attraction between A and B dipoles. This extra energy possessed by molecules by electric polarization is called ionic resonance energy.
Pauling Scale of Electronegativity Formula
Ionic resonance energy values are used by Pauling for the determination of electronegativity or electron affinity differences of elements in the periodic table.
The empirical formula used for the calculation of electronegativity of A – B molecule,
XA − XB = 0.208 √Δ + α
Where XA and XB = electronegativity of A and B and α = arbitrary constant which is nearly zero for chemical elements other than carbon, nitrogen, oxygen, fluorine, and helium.
Atomization Meaning
The quantity of heat required to produce one mole of atoms in the gaseous atomic state in the standard state is called the heat of atomization of the element It can be calculated from the spectrum measurement and some thermal data.
| Element | Atomization | Heat of atomization (kcal) |
| Carbon | C (s) → C (g) | 170.9 |
| Hydrogen | H2 (g) → 2 H (g) | 52.1 |
| Oxygen | O2 (g) → 2 O (g) | 59.6 |
| Nitrogen | N2 (g) → 2 N (g) | 113.0 |
| Chlorine | Cl2 (g) → 2 Cl (g) | 28.9 |
Calculation of Heat of Formation
The heat of formation is the heat change associated with the formation of 1 mole of a substance from its constituent elements in their stable forms. It is generally represented by ΔHf.
When the elements are in standard states, it is called standard heat of formation. For example, ΔHfo for CH4 = −17.9 kcal.
- A positive value of ΔHfo of a compound indicates that the compound is less stable than its constituents. It is called an endothermic compound.
- A negative sign refers to the compound being more stable. It is called an exothermic compound.
Bond Energy Calculation
The thermodynamics heat of formation and heat of atomization formula from the above table is used to calculate the bond energy of a molecule. Some workout examples are given below,
C-H bond Dissociation Energy
The heat of formation of methane = −17.9 kcal. The heat of atomization of solid carbon and four gaseous hydrogen atoms = 170.9 kcal and 4 × 52.1 kcal respectively.
| C (s) → C (g) | ΔHC = 170.9 kcal |
| 2 H2 (g) → 4 H (g) | ΔHH = 4 × 52.1 kcal |
| CH4 → C (s) + 4 H (g) | ΔHd = +17.9 kcal |
| CH4 → C (s) + 4 H (g) | ΔH = 397.2 kcal |
From the above data, the energy required to break four carbon-hydrogen bonds,
= 170.9 + (4 × 52.1) + 17.9 = 397.2 kcal
Therefore, the carbon-hydrogen bond enthalpy,
= 397.2/4 ≈ 99 kcal.
Such calculation shows that carbon-hydrogen bond enthalpy in a hydrocarbon like methane and ethane are the same but this is not strictly correct. The energy varies due to the linking and the stereochemistry of molecules.
How to Calculate Heat of Formation?
Automation and bond dissociation energy data are used during the calculation of the heat of the formation of a molecule.
For example, the formation of methyl alcohol from carbon, oxygen, and hydrogen,
C (s) + 2 H2 (g) + ½O2 (g) → CH3OH (l)
Atomization of 1 mole carbon = 170.9 kcal
For 2-mole hydrogen = 4 × 52.1 kcal
For half-mole oxygen = 59.6 kcal
Formation of 3 carbon-hydrogen bonds = (−3 × 99) kcal
For 1 C-O bond = −84 kcal
For 1 O-H bond = −110.5 kcal
Liquefaction of 1 mole CH3OH = −8.4 kcal
From the above data, the heat of the formation of liquid alcohol = − 61 kcal. If there exists one or more resonating structures, the resonance energy is also taken in the bond energy calculation formula.