Soil Contamination Pollution
Soil pollution or soil contamination is a part of land degradation causes by acid rain, excess and wrong use of agricultural fertilizer, insecticides ( the chemical compounds that kill insects), and herbicides (chemical substances that kill plants) to effects the natural soil of our environment. Petroleum, polynuclear hydrocarbon, and heavy metals are the most common substances which cause soil contamination and water pollution. All the soil contains different types of harmful or toxic polluted substances for human beings and other living cells. However, the natural concentration of such substances does not threaten the surroundings of our ecosystem. Soil contamination pollution effects or damages living cells, when the concentration of one or more of such toxic molecule is large enough. Acid rain water, waste disposal, accidental oil spills, and industrial, and agricultural activity are the main causes of soil pollution that have adverse effects on our environment in several ways.
Soil is an important natural resource that decides the diversity of life in an area. It is the outermost layer of the earth that takes a long period of time to form and provides support for the growth of plants. Therefore, we need to conserve soil or free it from pollution or contamination.
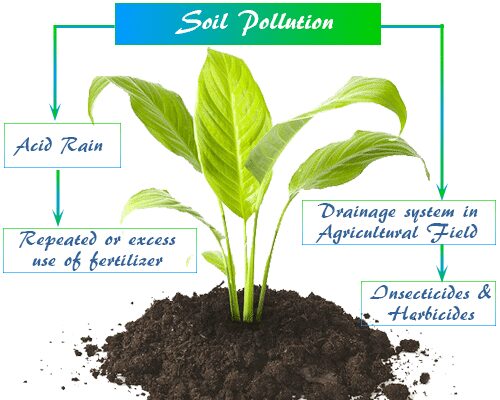
Causes of Soil Pollution
Soil is a mixture of small particles of rocks, bits of decayed living organisms (humus), and partially decomposed plant and animal matter (detritus). It also contains various microorganisms. Factors such as the removal of useful components and the addition of hazardous substances can cause soil contamination or pollution. The main factors and causes of soil pollution may include:
- Acidic or alkaline water molecules
- Repeated or excessive use of fertilizer
- Inadequate drainage systems in agricultural fields,
- Spraying of insecticides, and herbicides
The different causes and their adverse effects on soil are discussed below in this learning chemistry article.
Pollutants in Soil
The soil of our environment influences almost all the activities of our daily life but we fail to understand the importance of soil. Therefore, the soil gets polluted and effects our daily life in several ways.
Generally, soil pollution has harmful effects on human health, and the growth of plants by decreasing soil fertility, and changing the structure of the soil. Some major causes and adverse effects of soil pollution are given below,
Waste Disposal
Wastes are produced by everyday industrial and human activities such as washing clothes and excreting urine and farces. The discharge of large quantities of waste into rivers, lakes, and landfills causes water and soil pollution in our environment. Such types of pollutants have adverse effects on ground water and human health.
Acid Rain Water
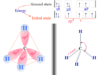
We know that the air of our environment contains gaseous sulfur dioxide (SO2) and nitrogen dioxide (NO2). Sulfur dioxide present in the air undergoes photolytic or catalytic oxidation to form sulfur trioxide (SO3). It reacts with rainy water or moisture to form sulfuric acid rain,
SO3 + H2O → H2SO4
Such sulfuric acid rain generally causes soil and water pollution.
Nitrogen dioxide (NO2) obtained from various natural sources and during the burning of fossil fuels also reacts with rainy water or moisture to form nitric acid (HNO3) rain. Nitrogen dioxide also catalyzes the conversion of ozone to oxygen.
The sulfuric acid and nitric acid formed by the above process come down to the atmosphere to damage standing crops or change the pH scale of soil.
However, atomic nitrogen is converted to suitable nitrates and nitrites by physical process lighting. The high temperature and pressure created in the air during lightning convert nitrogen into the oxide of nitrogen. They dissolve in rainwater to form nitric acid or nitrous acid and fall into the rain.
Excess Fertilizers
Repeated and excessive use of the same fertilizer pollutes the soil of our environment. Contamination of excess fertilizers increases the acidity of soil and some make the soil alkaline.
When we used ammonium sulfate [NH4(SO4)] fertilizer for the production of crops in the soil, again and again. The ammonium ion (NH4+) is used up by crops but the sulfate ion (SO4−2) gets contaminated into the soil responsible for increasing the acidity of the soil. The contamination of sulfate ions makes the soil highly acidic, hence the soil unfit for plant growth.
If we use sodium nitrate (NaNO3) or potassium nitrate (KNO3) again and again, the nitrate ion (NO3−) is used up by successive crops but sodium and potassium ions get accommodated into the soil. These fertilizers make the soil alkaline and hence the soil cannot be used for the high production of food.
Agricultural Activities
We use a lot of water for irrigation along with fertilizer, but there should be a good drainage system for the outlet of unused water. Otherwise, the soil becomes highly saline (salt-containing) which affects the growth of plants and is responsible for soil pollution. Therefore, the inadequate drainage system in the agricultural field is another source of soil pollution.
Use of Pesticides in Agriculture
When we speared different types of pesticides and herbicides to save the fruits and vegetable plants from harmful insects and herbs. Pesticides and herbicides enter into the living tissue of the growing plants in our environment.
Pesticides and herbicides cause soil pollution and toxic effects on our health. When we eat these grains, fruits, or vegetables, it may damage our heart, kidneys, etc. Therefore, we advise that before eating these types of polluted foods growing from soil contamination or pollution, they should be washed with a sufficient quantity of water.
How to Raise pH Level in Soil?
Soil acidity can be easily removed or the pH of the soil can easily rise by adding basic material like limestone or calcium oxide to neutralize the soil. Calcium carbonate or limestone is widely used because it is easy to handle and economically lower prices than other basic substances.
Soil Pollution Solutions
Soil contamination pollution can be prevented or minimized by the following methods,
- Reducing the emission of sulfur dioxide and nitrogen dioxide causes acid rain to increase soil acidity.
- The use of excess nitrate, sulfate, and phosphate fertilizer should be avoided because it increases or decreases the acidity of the soil.
- To minimize the use of synthetic detergents, properly recycle batteries and other harmful chemicals that cause soil pollution.
- Before throwing industrial wastes into the soil, they should be treated chemically to neutralize harmful substances present in them. These should be throwing the places that are authorized for them.
- Encourage the eco-friendly model for the industry, farming.
- Using proper drainage system for agriculture.
- Improving urban planning and wastewater treatment.
- Decreasing the use of harmful pesticides and herbicides that cause soil contamination pollution.