Ethylene Gas
Ethylene is a simple organic compound or unsaturated hydrocarbon that contains a double bond with the molecular formula C2H4 or CH2=CH2. It may be prepared by most of the general methods of preparation of alkenes or olefins. The most important laboratory method for the production of ethylene gas is heating ethanol with concentrated sulfuric acid (H2SO4). Two carbon atoms in ethane have the power to combine six hydrogen atoms. However, in the CH2=CH2 structure, four univalent hydrogen atoms are present and it is considered an unsaturated hydrocarbon in organic chemistry.
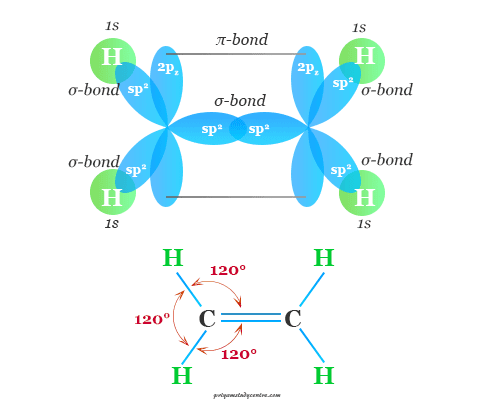
Structure of Ethylene
The excited-state electronic configuration of the carbon atom in the ethylene molecule is 2s1 2px1 2py1 2pz1. One s-orbital and two p-orbitals combine to form sp2 hybridized orbitals in the ethylene gas molecule. Therefore, the remaining 2pz orbitals of two carbon atoms used for the formation of pi-bonding in the CH2=CH2 molecule are shown in the below picture,
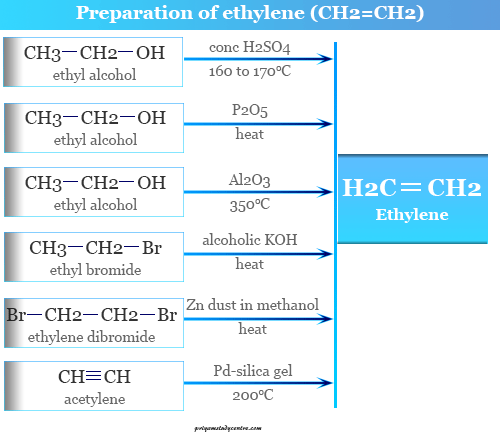
Production of Ethylene Gas
Huge quantities of ethylene are produced by the creaking of crude oil or petroleum.
- For the production of lower alkenes, the most suitable starting material is gas oil.
- However, for higher alkenes, the best process is paraffin wax or Fischer-Tropsch wax.
Ethylene from Ethyl Alcohol
It was prepared from ethanol by the action of concentrated sulfuric acid at 160 to 170 °C. Therefore, concentrated sulfuric acid dehydrated 1 molecule of water from ethanol to form CH2=CH2.
It was also prepared by the dehydration of ethanol by phosphorus pentoxide (P2O5) or alumina (Al2O3) at 350 °C.
Ethene from Ethyl Chloride
Ethylene may be prepared by the action of ethanolic potassium hydroxide on ethyl chloride or bromide.
1, 1, or 1, 2 dihalogen derivatives of ethane are dehalogenation by zinc dust in methanol to produce ethylene gas. This method is not useful for the preparation of CH2=CH2 but it is very useful for the purification of ethylene.
Hydrogenation of Acetylene
It may be prepared by hydrogenation of acetylene by palladium in silica gel at 200 °C.
Chemical Properties
It is a colourless gas with a boiling point of −105 °C. C2H4 is sparingly soluble in water but soluble in alcohol or ether. It burns in the air with a smoky luminous flame. When CH2=CH2 is heated with chlorine at 350 to 450 °C, it forms vinyl chloride.
Ethylene oxide is manufactured by passing CH2=CH2 and air under pressure over a silver catalyst at 200 to 400 °C.
It polymerized under high pressure and high temperature to form polyethylene or polythene. Polythene is also manufactured by the Ziegler process.
Chemical Reactions
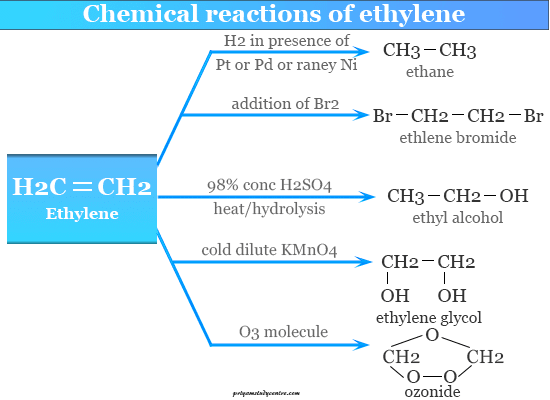
Hydrogenation of Ethylene
It is readily hydrogenated under pressure in the presence of chemical catalysts like platinum and palladium at room temperature.
Nickel catalyst is used under 200 to 300°C. Raney nickel is an effective catalyst at room temperature and atmospheric pressure.
Addition Reaction
CH2=CH2 forms an addition compound with chlorine or bromine. It also forms an addition compound with halogen acids.
For example, CH2=CH2 adds hydrogen bromide to form ethylene bromide. The order of reactivity of the addition of halogen acids is HI > HBr > HCl > HF. It is hydrated to ethanol by absorption of concentrated sulfuric acid followed by hydrolysis.
Hydroxylation of Ethylene
It is readily hydroxylated or adding hydroxyl groups to form glycols. By cold dilute alkaline permanganate solution, it is generally converted into cis-ethylene glycol.
Osmium tetroxide readily reacts with CH2=CH2 to form cyclic compounds like osmic ester. On refluxing osmic ester with ethanolic sodium hydrogen sulfate to form 1,2-glycol.
Ozonolysis Reaction
It adds ozone gas molecules to form an ozonide compound. The ozonide is oxidized by a silver oxide, hydrogen peroxide, or peracids to form formic acid.
Reduction of ozonide with zinc dust to form formaldehyde. Reduction may also be carried out with lithium aluminum hydride or sodium borohydride to form corresponding alcohol.
Uses of Ethylene Gas
- It is used for ripening fruits. Therefore, ripening fruits in the presence of ethylene gas are used for transporting fruits without any damage.
- It is generally used in medicine as an anesthetic, in the manufacture of mustard gas, and plastics (polythene, polystyrene), and in the extraction of rubber.
- It is used in metal fabrication for cutting or welding metal.
- It is also used as a refrigerant in LNG liquefaction plants.
- For the preparation of various organic solvents like glycol, dioxan, and cellosolves, we also used ethylene gas.