What is ozone gas?
Ozone gas is a triatomic allotrope of oxygen with the molecular formula O3. It is an important constituent of the upper atmosphere where the ozone layer is formed by solar uv radiation of very high energy.
It is a toxic blue gas freezing to a purple solid at −193 °C. It is one of the strongest oxidizing agents that oxidize silver (I) to silver (II). It is used for converting alkenes or olefins into aldehydes, ketones, or carboxylic acids.
Ozone molecules absorbed moderately high energy uv radiation to protect the living world from harmful ultraviolet rays.
Ozone depleting substances
- Nitrogen dioxide (NO2) from supersonic aircraft and industries catalyze the conversion of ozone to oxygen.
- Similarly, chlorofluorocarbons (CFC) photochemical decompose to give chlorine atoms which break the O3 molecule in a chain process.
It may be observed that the nitrogen dioxide and chlorine atoms are continually regenerated which causes to damage the O3 balances in the upper atmosphere.
Structure of ozone molecule
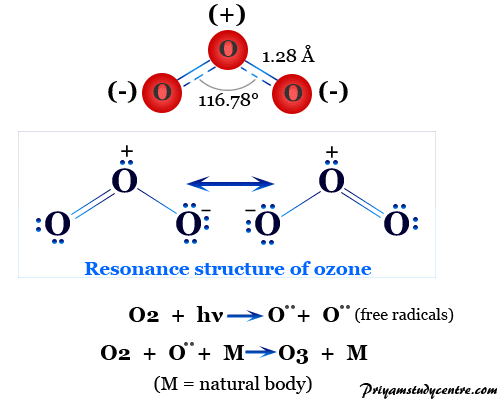
Ozone has a planar structure with O-O-O angle equal to 116.78° and O-O chemical bond lengths equal to 1.28 Å. The central oxygen atom in the molecule is sp2 hybridized with one lone pair.
The valence bond theory describes the two resonating forms of O3 molecule, each has a single bond on one side and a double bond on another side. It is a polar molecule with a dipole moment of 0.53 D.
If we count the valence electron, the molecule has a total of 6×3 = 18 valence electrons of which four are used for forming sigma bonds. The central oxygen has one electron pair and the end oxygen atom has two electron pairs each. The remaining four-electron in the ozone molecule is used for forming pi-bonding.
Properties
Physical properties of ozone
Ozone is a faint blue colour gas with a typical pungent smell. It is slightly soluble in water but more soluble in non-polar solvents like carbon tetrachloride.
| Properties of ozone gas | |
| Molecular formula | O3 |
| Systematic IUPAC name | Trioxygen |
| Appearance | Colourless to pale blue gas |
| Molar mass | 47,997 g/mol |
| Density | 2.144 mg/cm3 at 0 °C |
| Dipole moment | 0.53 D |
| Melting point | −192.2 °C |
| Boiling point | −112 °C |
| Solubility in water | 1.05 g/l at 0 °C |
Chemical properties of ozone
- Both the liquid and gaseous forms of O3 are diamagnetic in nature.
- The pure liquid is dangerously explosive chemicals.
- In acid solution, the potential of ozone is lower than those of a few oxidizing agents like fluorine, perxenate, and atomic oxygen.
- It may be estimated iodometrically by titrating iodine liberated form of a solution buffered with boric acid.
- Ozone reacts with dry powdered alkali to form red-brown paramagnetic ozonides. The stability of ozonide decreasing in the sequence of decreasing the cationic size (cesium > rubidium > potassium > sodium and barium > strontium > calcium) or increasing polarizing power. Lithium ozonide is known as amine like LiO3, 4NH3.
Formation of ozone
It may be formed artificially or naturally. The stratospheric ozone layer is formed naturally through the interaction of solar ultraviolet (uv) radiation with molecular oxygen (O2).
Artificially, the gas may be prepared by the silent electric discharge of oxygen and streaming out the gas quickly to avoid conversion.
It was also obtained by the anodic oxidation of a concentrated aqueous solution of perchloric acid at 50 °C. Pure ozone gas may be obtained by fractional distillation of a blue liquid mixture of O3-O2.