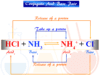
What is acid rain?
Acid rain or any other form of precipitation that causes mainly by pollutants like sulfur dioxide or nitrogen dioxide has adverse effects on the environment or humans. Nitric acid or sulfuric acid rain comes down in the environment by raindrops, snow, fog, hail, or even dust.
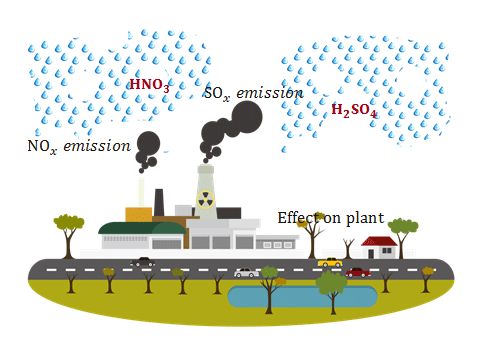
It can adverse effects on plants, aquatic life, soil, and archeological specimens like bridges, stone buildings, and statues of our environment.
Most of the water has a pH scale range between 6.5 to 8.5 but the pH value of acid rain is less than that of such ranges. Acid rain causes several types of pollution such as soil pollution and water pollution in our enviorment. Therefore, we need to find scientific solutions to minimize the effects of such types of pollution caused by acid rain.
Acid rain sources
It is formed mainly by the emission of SOx and NOx from different types of sources like coal, oils or automobile engine burning, etc.
Natural rainwater is also slightly acidic (pH scale = 5.6) because it contains some atmospheric carbon dioxide. It reacts with water to form the carbonic acid solution.
Sources of sulfur dioxide
- Sulfur dioxide and nitrogen dioxide are the major pollutants of our environment that cause air, water, and soil pollution. Sulfur dioxide is released by burning oil, and the coal industry reacts with rainwater to form sulfuric acid. It damages the plants, animals, and human life in our environment.
- About 200 million tonnes of sulfur dioxide are released over the world from different types of industries. Equal amounts of SO2 are released by different types of natural processes.
Sulfuric acid rain
Sulfur dioxide is partial oxidation to form sulfur trioxide in the air by the photolytic or catalytic process initiated by ozone gas, hydrocarbon, nitrogen oxides, and soot or dust. This sulfur trioxide reacts with rainwater or moisture and gives sulfuric acid.
This acid comes down the atmosphere in the form of sulfuric acid rain or acid snow. It causes mainly water pollution and soil pollution.
| 2SO3 + O2 → 2SO3 |
| SO2 + O3 → SO3 + O2 |
| SO3 + H2O → H2SO4 |
Nitric acid rain
Sources of nitrogen dioxide
The smoke released by the automobile’s engines contains nitrogen dioxide that causes acid rain and the greenhouse effect. This smoke is obtained by the thermodynamic combustion of petrol or diesel in different types of engines. Many chemical plants like explosives or nitrogenous fertilizers production plants produce nitrogen dioxide.
Large amounts of sulfur or nitrogen dioxide are also emitted from power plants and many industrial processes. These mixtures of gases contain sulfur dioxide or nitrogen dioxide It is called flue gases.
Acid rain reaction
The nitrogen dioxide produces from the above sources reacts with the rainwater or moisture in the presence of oxygen or ozone to form nitric acid rain or snow.
| 2NO + O2 → 2NO2 |
| NO + O3 → NO2 + O2 |
| NO2 + NO3 → N2O5 |
| N2O5 + H2O → 2HNO3 |
Effects of acid rain on the environment
It serves many harmful impacts on animal, plant, and fish life.
- Acid rain has served ecological impacts. It gradually eating up the limestone and marble of the bridges, buildings, statues, monuments, tombstones, and other cultural artifacts.
- Due to their corrosive nature, these pollutants decolorize building materials like limestone, marble, roof slate, and metals.
- Fabrics, leather, paper, and paints undergo fading of their color in the presence of SO2 and NO2.
- The negative effect of acid deposition increases the acidity of the soil and lakes water. This changes the solubility of the different metal elements salt in soil mainly the aluminum salt in the soil.
- The effects of acid rain are seen in the fish, plants, and human life of our environment. With the increasing acidity of the water, the fertility of fishes decreases, or young fishes are dying.
Effects of acid rain on humans
- It can not affect or harm humans directly. It increases the concentration of many dangerous metals or particles through acidic water. When we drink such water, it is transported to our body which affects our lungs and kidney.
- It also makes the lakes so acidic that they can no longer support drinking or bathing.
- The formation of acid rain reduces the formation of agricultural crops which we used in our daily life.
- The nitrogen dioxide which creates acid rain promotes the formation of ground-level ozone gas. It causes lung problems for human beings. It also has adverse effects on our skin, cells, and the neuronal system of the body.
Solutions of acid rain
- For the solution of acid rain, increasing the use of renewable energy like wind, solar energy, and hydroelectric energy unit for the source of electricity.
- Using requisite technology for reducing the emission of NOx and SOx from the thermal power plant or different production factories.
- Decreasing the use of fossil fuels in our daily life to decrease this type of pollution.
- Using technology in vehicles for reducing the emission of NOx. Therefore, modern technology used in vehicles or thermal power plants reduces the harmful effects of acid rain on humans or the environment.
How to reduce nitrogen dioxide levels?
Nitrogen dioxide can be controlled by reducing with air in the presence of the platinum catalyst produces nitrogen and ammonia molecules. Therefore, before this enters the atmosphere this process may be applied.
How to remove sulfur dioxide from air?
The density of sulfur dioxide decreases by treating the flue gases with the slurry of calcium carbonate which absorbed the SO2 gases due to the formation of CaSO3. But the process is quite economical.
Sulfur dioxide may also be reduced partially by desulphurization of coal and Naptha or using natural gas in place of coal. But this technical information for the solutions of acid deposition facts is not fully utilized in practice due to many causes.