Types of Hydrocarbons with Examples
Hydrocarbon (aliphatic, aromatic, or polynuclear hydrocarbon) is a type of organic compound that processes hydrogen and carbon in the entire molecular formula. Hydrocarbons (alkanes, alkenes, and alkynes) contain at least one single, double, or triple bond in two adjacent carbon atoms. According to the reactivity, hydrocarbons are classified into two main types, saturated and unsaturated hydrocarbons. When organic compounds formed by two or more carbon atoms by a single chemical bond are called saturated hydrocarbons. In organic chemistry, the compounds containing at least one pair of adjacent carbon atoms linked by multiple chemical bonds are called unsaturated hydrocarbons. Several examples of saturated or unsaturated types of hydrocarbons come from natural sources named aliphatic hydrocarbons.
Structure of Hydrocarbons
In the excited state, the carbon atom can promote an electron from 2s-orbital to vacant 2pz-orbital. Therefore, the excited state electronic configuration of the carbon atom in a hydrocarbon molecule is
1s2 2s1 2px1 2py1 2pz1
The 2s and 2p orbitals of carbon atoms hybridize to form sp3, sp2, and sp hybrid orbitals. From the hybridization of carbon atoms, we can predict the structure and geometry of hydrocarbon molecules.
sp3 Hybridization Structure
When one 2s orbital and three p orbitals hybridize, they form four equivalent sp3 hybrid orbitals. The hybrid orbitals produced by sp3 hybridization contain a tetrahedral structure.
When four hybridized orbitals of carbon atoms mix with the hydrogen atom, it produces a saturated organic compound with a tetrahedral structure. For example, the saturated hydrocarbon methane (CH4) has a tetrahedral shape with an H−C−H bond angle of 109.5°.
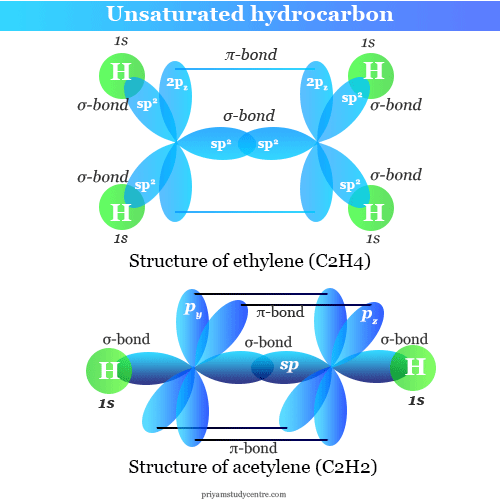
sp2 Hybridization Structure
Mixing one 2s and two 2p (2px and 2py) orbitals of carbon atoms gives three equivalent sp2 hybrid orbitals pointing to the xy plane. The remaining orbital is the undisturbed 2pz orbital. The hybrid orbitals produced by sp2 hybridization contain a trigonal planar or triangular structure with a bond angle of 120°.
For example, in ethylene, the three co-planner sp2 hybrid orbitals form three σ bonds and two pz orbitals form one π bond (pi-bond). The pz electrons in π-bond are called mobile electrons, or unsaturated electrons.
sp Hybridization Structure
In sp hybridization, only one 2s electron and one 2px electron are hybridized to form two equivalent colinear orbitals. The remaining py and pz orbitals are undisturbed.
For example, in acetylene, each carbon atom contains an sp hybrid orbital and two unhybridized 2py and 2pz orbitals. The two ends of colinear sp hybrid orbitals overlap to form a strong σ bond between the two adjacent carbon atoms. The remaining two ends of sp hybrid orbitals attached two hydrogen atoms by σ bonding.
The unhybridized py orbital of one carbon atom forms a weaker π bond (pi-bond) with the py orbital of another carbon atom. Another π bond (pi-bond) is formed between the two pz orbitals of two adjacent carbon atoms.
Chemical Properties of Hydrocarbon
The solubility of hydrocarbons shows that hydrocarbon molecules are almost insoluble in water but rapidly soluble in alcohol or ether solution. When concerned with alkanes, the attractive forces are only weak Van der Waals forces.
The dipole moment of hydrocarbon like all alkanes whether straight or branched-chain will be zero. Zero polarity of these hydrocarbons is observed due to balancing the C-H covalent bond by the remaining alkyl functional group.
The position of the electromagnetic spectrum region of the C-H group depends on the carbon atom. Therefore, the stretching frequency region of hydrocarbons is in the following order,
primary carbon > secondary carbon > tertiary carbon
−CH3 > (CH3)2CH− > (CH3)3C−
Classification of Hydrocarbons
According to the reactivity, hydrocarbons are classified into two main classes,
- Saturated hydrocarbons: The term saturated means the valency of carbon saturated by single covalent bonds.
- Unsaturated hydrocarbons: Unsaturated hydrocarbons contain at least one double or triple bond along with single bonds.
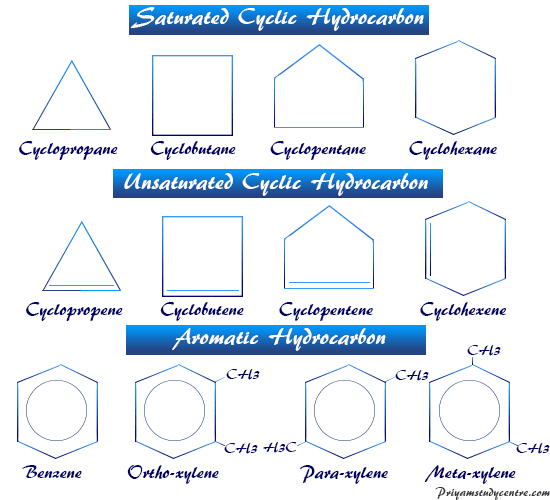
According to the structure in chemistry, hydrocarbons are classified into two types, open-chain and cyclic or closed-chain saturated and unsaturated organic compounds present in our environment.
Saturated Hydrocarbon
The list of organic compounds that contain two or more carbon atoms linked by only a single chemical bond is named by the term saturated hydrocarbons. They are open-chain and cyclic saturated hydrocarbons.
Alkenes or Paraffins
All the open-chain saturated hydrocarbons (alkenes or paraffins) are formed by single covalent carbon and hydrogen bonding. Therefore, alkenes or paraffins are open-chain saturated hydrocarbons that contain the general molecular formula CnH2n+2. Here, n = number of carbon atoms in one molecule of hydrocarbon.
- If there is only one carbon atom, the molecular formula should be CH4 (methane).
- Similarly, if there are two carbon atoms, the molecular formula of saturated hydrocarbon is C2H6 (ethane).
The name and general molecule formula of the first twenty alkanes or paraffin are given below in the table,
| Number of Carbon Atom | Name of Alkane | Formula | Number of Carbon Atom | Name of Alkane | Formula |
| 1 | Methane | CH4 | 11 | Undecane | C11H24 |
| 2 | Ethane | C2H6 | 12 | Dodecane | C12H26 |
| 3 | Propane | C3H8 | 13 | Tridecane | C13H28 |
| 4 | Butane | C4H10 | 14 | Tetradecane | C14H30 |
| 5 | Pentane | C5H12 | 15 | Pentadecane | C15H32 |
| 6 | Hexane | C6H14 | 16 | Hexadecane | C16H34 |
| 7 | Heptane | C7H16 | 17 | Heptadecane | C17H36 |
| 8 | Octane | C8H18 | 18 | Octadecane | C18H38 |
| 9 | Nonane | C9H20 | 19 | Nonadecane | C19H40 |
| 10 | Decane | C10H22 | 20 | Eicosane | C20H42 |
A set of organic compounds in which the members differ in composition from one another by CH2 is called a homologous series. The individual members are called homologues. From the above table, if we examine the formula of various alkanes, we find that the formula of each individual alkane differs from its neighbor by CH2.
Cyclic Saturated Hydrocarbons
Cycloalkanes are examples of cyclic saturated types hydrocarbons with the general molecular formula CnH2(n+1−r), where r = number of ring/rings in one saturated hydrocarbon molecule.
For monocyclic hydrocarbon, the general molecular formula = CnH2n, because r = number of ring = 1. Cyclopropane (C3H6), cyclobutane (C4H8), cyclopentane (C5H10), and cyclohexane (C6H12) are examples of monocyclic saturated types of hydrocarbons that contain only one cyclic ring in their structure.
Unsaturated Hydrocarbon
The compound that contains at least one multiple bond in adjacent carbon atoms is called an unsaturated hydrocarbon. They are further classified into two classes alkenes or olefins and alkynes.
Alkenes or Olefins
The open-chain hydrocarbons that have at least one double bond along with single bonds are called alkenes or olefins. A double bond in alkenes is called an olefinic bond or ethylenic bond and is formed by the sharing of two pairs of electrons between two carbon atoms.
The general molecular formula of alkene = CnH2n. Therefore, when an alkene contains two carbon atoms or n = 2, its molecular formula = C2H4 (ethene). Similarly, when n = 3, the molecular formula of alkene or olefin = C3H6 (propene). In this way, we can write the molecular formulas of other alkenes or olefins.
| Name of Alkene | Number of carbon Atom | Molecular Formula |
| Ethene | 2 | C2H2×2 = C2H4 |
| Propene | 3 | C3H3×2 = C3H6 |
| Butene | 4 | C4H4×2 = C4H8 |
| Pentene | 5 | C5H5×2 = C5H10 |
| Hexene | 6 | C6H6×2 = C6H12 |
| Heptene | 7 | C7H7×2 = C7H14 |
| Octene | 8 | C3H3×2 = C8H16 |
| Nonene | 9 | C3H3×2 = C9H18 |
| Decene | 10 | C3H3×2 = C10H20 |
Alkynes
The unsaturated hydrocarbons which contain at least one triple bond or acetylenic bond along with single bonds are called alkynes. A triple bond in alkyne molecules is formed by the sharing of 3 pairs of electrons between two adjacent carbon atoms.
The general molecular formula of alkynes = CnH2n−2
- For example, if an alkyne has two carbon atoms (n = 2), then its formula,
= C2H2×2−2
= C2H2 (ethyne or acetylene) - When there are three carbon atoms in the alkyne, the molecular formula of said alkyne,
= C3H2×3−2
= C2H4 (propyne)
Many other organic compounds such as acetaldehyde, formaldehyde, and acetone contain multiple bonds between adjacent carbon and oxygen atoms but it is not a condition for unsaturation.
Homologous Series
A set of similarly constituted organic compounds in which the members present have the same functional group and similar chemical properties and any two successive members in a particular set differ in their molecular formula by −CH2− unit is called a homologous series.
- Alkane series contain CH4, C2H6, C3H8, C4H10, C5H12, C6H14, etc.
- Alkene series contain C2H4, C3H6, C4H8, C5H10, C6H12, etc.
- Alkyne series contain C2H2, C3H4, C4H6, C5H8, C6H10, etc.
Characteristics of Homologous Series
- All the members of a homologous series can be represented by the same general molecular formula.
- Any of the two adjacent homologues differ by one carbon atom and two hydrogen atoms.
- The chemical properties of all organic compounds present in a homologous series are very similar.
- A gradual change in physical properties (melting and boiling point) in a homologous series can be seen with the increasing molecular mass.
- The difference between the molecular masses of any two adjacent members of a homologous series is 14 u.
Cyclic Hydrocarbon
When the carbon and hydrogen atoms form a closed ring structure, it is called carbocyclic or homocyclic compounds. The cyclic compounds show aliphatic characteristics named alicyclic hydrocarbons. The saturated types of cyclic hydrocarbons take the name of the corresponding open-chain hydrocarbons, proceeding by the suffixes cyclo.
Cycloparaffins or Cycloalkanes
The cyclic saturated organic compounds are collectively called cycloparaffins or cycloalkanes. The general molecular formula of cycloalkanes = CnH2(n−r), where r = number of ring/rings in a cyclic hydrocarbon molecule.
- The saturated alicyclic hydrocarbons which contain one ring in their structure have the general molecular formula CnH2n (same as that of alkenes).
- When the saturated alicyclic hydrocarbons contain two rings, the general molecular formula of the compound = CnH2n−2.
Cycloalkenes or Cycloolefin
A cycloalkene or cycloolefin is a type of unsaturated hydrocarbon that contains a closed ring of carbon atoms with one or more double bonds but has no aromatic character. Cyclobutene and cyclopentene are the most common cycloalkenes that are used for the production of polymer chains.
In learning organic chemistry, the general molecular formula of monocyclic cycloalkenes with only one double bond is CnH2(n−r).
For cyclopropene n = 3 and r =1
∴ The molecular formula of cyclopropene,
= C3H2(3−1)
= C3H4
For cyclobutene n = 4 and r =1
∴ The molecular formula of cyclobutene,
= C4H2(4−1)
= C4H6
In cyclopentene, n = 5 and r =1
∴ The molecular formula of cyclopentene,
= C5H2(5−1)
= C4H8
For cyclohexene n = 6 and r =1
∴ The molecular formula of cyclohexene,
= C6H2(6−1)
= C4H10
Aliphatic and Aromatic Compounds
With the development of learning chemistry, organic compounds are divided mainly into two types which are pronounced as aliphatic and aromatic hydrocarbons.
Aliphatic Hydrocarbons
The aliphatic naming of hydrocarbon is given from fatty acids but today these compounds are known as open-chain compounds. The closed chain or cyclic compounds that do not show aromatic character are also included in the class of aliphatic hydrocarbons.
Therefore, we can define, aliphatic hydrocarbons are a class of organic compounds containing hydrogen and carbon atoms linked together by single, double, or triple covalent bonds to form chains or non-aromatic ring-like structures.
Several examples of saturated or unsaturated types of hydrocarbons come from natural sources named aliphatic hydrocarbons. Some common saturated and unsaturated hydrocarbons with their name and molecular formula are given below in the table,
| Number of Carbon Atoms | Aliphatic hydrocarbons | |
| Saturated | Unsaturated | |
| 1 | methane (CH4) | |
| 2 | ethane (C2H6) | ethene (C2H4), ethyne (C2H2) |
| 3 | propane (C3H8), cyclopropane (C3H6) | propene (C3H6), propyne (C3H4) |
| 4 | methylpropane (C4H10), butane (C4H10), cyclobutane (C4H8) | Butene (C4H8), butyne (C4H6), cyclobutene (C4H6) |
| 5 | pentane (C5H12), dimethylpropane (C5H12), cyclopentane (C5H10) | pentene (C5H10), pentyne (C5H8), cyclopentene (C5H8) |
| 6 | hexane (C6H14), cyclohexane (C6H12) | hexene (C6H12), hexyne (C6H10), cyclohexane (C6H10) |
| 7 | heptane (C7H16), cycloheptane (C7H14) | heptene (C7H14), heptyne (C7H12), cycloheptene (C7H12) |
| 8 | octane (C8H18), cyclooctane (C8H16) | Octene (C8H16), octyne (C8H14), cyclooctene (C8H14) |
Aromatic Hydrocarbons
The aromatic type name of hydrocarbon is obtained from the Greek prefixes aroma meaning fragrant smell. The detailed characteristics show that the aromatic hydrocarbon compound contains a ring with the aromatic property.
Originally, the term aromaticity was used to describe all compounds that had the properties of benzene or contained benzene ring or a condensed system of benzene rings.
In another way, an aromatic molecule is an organic cyclic molecule that contains a planar system and (4n+2)π electrons. For benzene, n = 1, hence the molecule has six π (pi) electrons with a planner system.
Most of the hydrocarbon molecules are related to the benzene ring or derivative of benzene. The cyclic benzenoid compound has aromatic properties but the properties of these compounds are different from the alicyclic hydrocarbon molecules.
Hydrocarbon Sources
Natural crude oil or mineral oils are the main sources of aliphatic, aromatic, and cyclic hydrocarbons like methane, ethane, propane, cycloalkene, etc.
Natural gas is another source of hydrocarbons. It defines large quantities of gaseous substance with undissociated liquid petroleum fuel used as the daily energy source.
- When the natural gas does not contain hydrocarbons above ethane is called lean or dry gas.
- If it contains the higher member of hydrocarbons is called rich or wet gas.
What are the Components of Crude Oil?
Crude petroleum oil of our earth’s environment contains mainly gases with naturally occurring hydrocarbon in the forms of liquified or solid dissolved in liquid solvents.
The density and composition of crude petroleum oil vary with the locality of occurrence. All saturated organic compounds are mainly liquid hydrocarbons. Therefore, crude petroleum oil contains a list of isoparaffinic alkanes, cycloalkenes or naphthenes, and aromatic or polynuclear aromatic hydrocarbons.
In addition to the hydrocarbon, there are also known derivatives of hydrocarbons containing oxygen, nitrogen, sulfur, and crystalline solid metallic atoms.
Hydrocarbons in Petroleum
Naturally, crude oil is classified into two types, paraffinic and asphaltic which contain hydrocarbon compounds like alkane and naphthalene.
- According to this classification crude oil from Pennsylvania, Iran, and Rumania contain paraffinic type.
- From Baku, Venezuela is an asphaltic type of hydrocarbon.
- Texas, Mexico, and Oklahoma is an intermediate type of hydrocarbon.
Natural Gas Composition
The term natural gas means the total quantity of gaseous hydrocarbon present in used liquid gases. Therefore, the main component of natural gas varies according to their sources.
Natural gas consists chiefly of the first six alkanes. The percentage of occurrence decreases with increasing molecular weight.
Fractional Distillation of Natural Gas
The hydrocarbon separated from natural gas by fractional distillation under pressure is used for different energy generation purposes in our daily lives.
Oxidation of natural gas under controlled conditions produces a mixture of organic aldehyde, acid, acetone, and alcohol. We use these organic compounds in the different chemical industries.
Hydrocarbons are obtained when natural gas combustion at about the temperature of 200 to 300°C with 1-200 atm pressure in the presence of a chemical catalyst. Most commonly we produced methane, ethane, propane, butane, pentane, and hexane by this process.
Cracking of Hydrocarbon
The thermal bond dissociation of organic molecules like hydrocarbon is known as pyrolysis. Thermal creaking produces a list of hydrocarbons or classes of alkanes from the higher members of the alkane family.
Alkanes decomposed to lower alkanes by specific heat like 500°C to 600°C. The product obtained from a given alkane depends on, structure, applied pressure, and used catalyst.
- The thermal decomposition of a list of hydrocarbons like decane given lower alkane octane and ethylene.
- If we use suitable catalysts, alkanes containing six or more carbon atoms are cyclized. Therefore, in the presence of the catalyst platinum, hydrocarbon decane is cyclized to form benzene.
Cracking of Petroleum
Organic fuels like petroleum oil creaking are used for the extraction of the most common list of liquid hydrocarbons like aliphatic, aromatic, and polyaromatic hydrocarbons.
For example, the most important list of hydrocarbons like methane, ethane, ethane, propane, butane, and isobutene is obtained from organic fuels that contain up to four carbon atoms in their molecular formula.
Frequently Asked Questions (FAQs)
What is a hydrocarbon simple definition?
Hydrocarbon can be simply defined as an organic compound containing carbon and hydrogen. For example, methane (CH4), ethane (C2H6), ethylene (C2H4), acetylene (C2H2), benzene (C6H6), etc.
What is an homologous series?
Homologous series is a set of similarly constituted organic compounds in which the members present have the same functional group and similar chemical properties and any two successive members in a particular set differ in their molecular formula by −CH2− unit.
For example, if we examine the formula of various alkanes, we find that the formula of each individual alkane differs from its neighbor by −CH2− unit.
How hydrocarbons are classified?
Earlier in the development of organic chemistry, hydrocarbons were classified into two types aliphatic and aromatic. Such classification is done based on the properties and sources of hydrocarbon molecules.
Today, we classify hydrocarbons based on their structure. Saturated and unsaturated hydrocarbons are the two main classes of hydrocarbons. They are also open-chain and cyclic organic compounds. Therefore, the 4 main types of hydrocarbons are alkanes or paraffins, alkene or olefins, alkynes, and aromatic hydrocarbons.
What are unsaturated hydrocarbons?
Unsaturated hydrocarbons are the class of hydrocarbons that contain at least one double or triple bond along with single bonds. Alkenes or olefins and alkynes are examples of the two sub types of unsaturated hydrocarbons.