Adenosine Triphosphate (ATP)
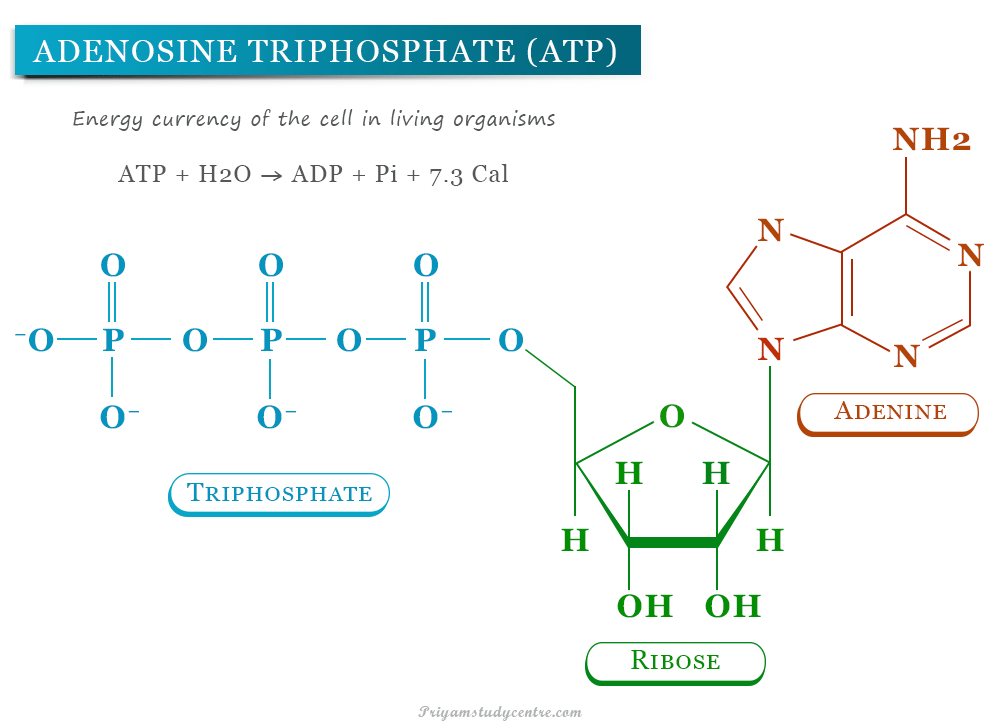
Adenosine triphosphate (ATP) in biology is a unique and most important high-energy biomolecule in living cells. The structure of adenosine triphosphate contains an adenine, a ribose, and a triphosphate moiety. ATP is a high-energy compound due to the presence of two phosphoanhydride bonds in a triphosphate unit. Adenosine triphosphate (ATP) is an energy currency of the cell in living organisms. During the metabolic cycle, adenosine triphosphate (ATP) converts either to adenosine diphosphate (ADP) or adenosine monophosphate (AMP). It is also a precursor to nucleic acids such as deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). Nucleoside monophosphate contains only one phosphate moiety (AMP, TMP). However, the addition of second and third phosphate groups to the nucleosides forms nucleoside diphosphate (ADP) and nucleoside triphosphate (ATP).
The energy-rich carbohydrates, fatty acids, and amino acids undergo a series of electron transport reactions and get oxidized to form carbon dioxide and water. The reducing equivalents from various intermediates are transferred to coenzymes nicotinamide adenine dinucleotide (NAD+) and flavin adenine dinucleotide (FAD) to form NADH and FADH2. The latter two reduced forms of coenzyme pass through the electron transport chain or respiratory chain reaction.
A significant amount of free energy is lost during such electron transport chain reactions. A part of such free energy is used to generate ATP from ADP and Pi. When NADH is oxidized, about 35% of energy is trapped in the form of 2.5 ATP and the remaining is lost as heat energy. Such heat is necessary to maintain body temperature.
It must be remembered that the life of higher animals is absolutely dependent on the supply of oxygen for electron transport reaction and generation of adenosine triphosphate.
Structure of Adenosine Triphosphate (ATP)
The chemical formula of adenosine triphosphate (ATP) is C10H16N5O13P3. Therefore, the ATP molecule contains carbon, nitrogen, hydrogen, oxygen, and phosphorus in its molecular structure with a molar mass of 507.18 g/mol. The density of the disodium salt of adenosine triphosphate is 1.04 g/cm3.
In biology, adenosine triphosphate (ATP) contains an adenine, a ribose, and a triphosphate moiety in its structure. During various biochemical reactions related to metabolism adenine and ribose groups remain unchanged but the triphosphate can be converted to diphosphate (ADP) and monophosphate (AMP).
The salts of adenosine triphosphate (ATP) is a colorless solid that soluble in water at the pH level of 6.8 to 7.4. Above the pH level, it hydrolyzed to form ADP and phosphate. In living cells, the concentration of ATP is much higher than the concentration of ADP.
ATP ADP Cycle
The hydrolysis of adenosine triphosphate releases a large amount of energy that is used by living cells to perform various biological reactions.
ATP + H2O → ADP + Pi + 7.3 Cal (30.5 kJ)
ATP + H2O → AMP + PPi + 10.9 kcal (45.6 kJ)
The energy liberated is utilized for various biological processes like muscle contraction, active transport, circulation of blood, etc. It can also act as a donor of high-energy phosphate to low-energy biomolecules, to make it energy-rich. Similarly, ADP can accept high-energy phosphate from compounds such as creatine phosphate, processing higher free energy content to form adenosine triphosphate.
Adenosine triphosphate (ATP) generally serves as an immediately available energy currency of the living cell which is constantly being utilized and regenerated through a cycle. The ATP ADP cycle is the fundamental basis of energy exchange biochemical reactions in living systems. It provides an energy link between catabolism and anabolism in the biological system.
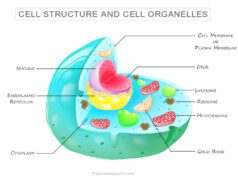
The mitochondria are the center for metabolic oxidative reactions to generate reduced coenzymes NADH and FADH2. They have utilized in electron transport chain reaction to liberater energy in the form of adenosine triphosphate. Therefore, mitochondria are also called power house of the cell.
Transport of Electrons
The transport of electrons from the redox pair NAD+/NADH) to the redox pair ½O2/H2O can be represented by the following equation,
½O2 + NADH + H+ (hydrogen ion) → H2O + NAD+
The redox potential difference between these two redox pairs is (0.82 + 0.32) = 1.14 V. It is equivalent to the energy of 52 Cal/mol. However, only 2.5 ATP is synthesized in the electron transport chain when NADH is oxidized. It is equivalent to the 18.25 Cal energy.
The efficiency of energy conservation during such biochemical reactions,
= 18.25 × 100/52 = 35.10%
Therefore, when NADH is oxidized, about 35% of energy is trapped in the form of 2.5 ATP and the remaining is lost as heat energy.
ATP Synthesis
Adenosine triphosphate (ATP) is mostly synthesized in two ways:
- Oxidative phosphorylation
- Substrate level phosphorylation
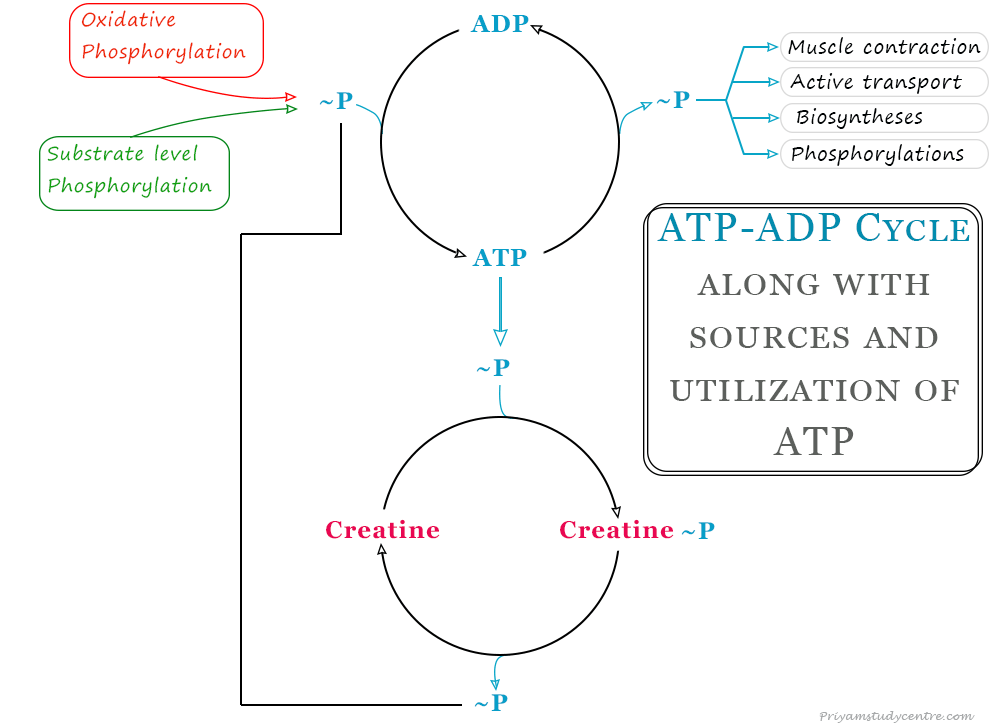
Oxidative Phosphorylation
Oxidative phosphorylation is the major biological source of ATP in aerobic cells of living organisms. It is linked with the mitochondrial electron transport chain (ETC) reaction. Therefore, oxidative phosphorylation is a process of synthesizing adenosine triphosphate from ADP and Pi coupled with the electron transport chain reaction. The inner mitochondrial membrane is the site where oxidative phosphorylation generally occurs.
Several hypotheses can be used to explain the process of Oxidative phosphorylation. The most common and important hypotheses are,
- Chemical Coupling: This hypothesis was given by Edward Slater in 1953. According to the chemical coupling hypothesis, a series of phosphorylated high-energy intermediates are produced which are utilized for the synthesis of adenosine triphosphate (ATP).
- Chemiosmotic: Such a widely accepted mechanism was proposed originally by Peter Mitchell in 1961. It generally explains how the transport of electrons in the respiratory chain is effectively utilized for the production of ATP from ADP + Pi.
- Rotatory motor model of ATP generation: Paul Boyer in 1964 proposed that a conformational change in the mitochondrial membrane proteins helps for the production of adenosine triphosphate. Now it is also called the engine driving model or binding change model.
Substrate Level Phosphorylation
Adenosine triphosphate (ATP) can be directly synthesized during the metabolism of substrate by oxidation. High-energy compounds such as phosphoenolpyruvate and 1, 3-phosphoglycerate (intermediates produced during glycolysis), and succinyl CoA (produced during the citric acid cycle) can transfer high-energy phosphate during the production of ATP.
The energy-rich compounds phosphocreatine or creatine phosphate are the storage forms of phosphates. It is stored generally in vertebrate muscles and the brain in the human body.
Adenosine Triphosphate Production
Adenosine triphosphate can be produced during two biochemical conditions, aerobic condition and anaerobic condition.
Aerobic Conditions
The production of adenosine triphosphate in non-photosynthetic eukaryotes occurs mostly in mitochondria. The dephosphorylation of ATP and rephosphorylation of ADP and AMP generally occur in aerobic metabolism. In aerobic conditions, adenosine triphosphate or ATP can be produced most commonly in three pathways glycolysis, citric acid cycle, and beta oxidation.
Glycolysis
Glycolysis is the degradation of glucose to pyruvate generating 8 Adenosine triphosphate (ATP). Under anaerobic conditions, 2 ATP are synthesized while under aerobic conditions, 7 ATP are synthesized.
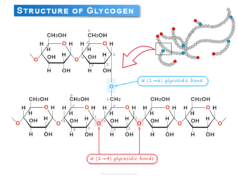
When glycolysis occurs from glycogen, one more ATP molecule is generated. During such a biochemical process, no ATP is consumed for the activation of glucose. Therefore, in anaerobic glycolysis, 3 ATP is produced from glycogen and it is more advantageous than glucose.
The three enzymes namely hexokinase, phosphofructokinase, and pyruvate kinase catalyze the irreversible reactions and regulate glycolysis.
Citric Acid Cycle
The citric acid cycle, or Kerb’s cycle, or tricarboxylic acid (TCA) cycle is the most important metabolic pathway for the energy supply to the body. Therefore, most of the adenosine triphosphate or ATP synthesized in our body goes through Kerb’s cycle.
The citric acid cycle essentially involves the oxidation of acetyl CoA to carbon dioxide and water. During the oxidation, it utilizes about two-thirds of the total oxygen consumed by the body.
Beta Oxidation
The fatty acids or lipids in the human body are mostly oxidized by beta-oxidation. It may occur on the β-carbon atom of the fatty acids. The beta-oxidation of fatty acids can carried out in three stages:
- Activation of fatty acids occurs in the cytosol.
- Transport of fatty acids into mitochondria.
- Beta-oxidation happens on the mitochondrial matrix.
Fatty acids oxidize most of the tissues in the body to form energy currency or adenosine triphosphate (ATP). Fatty acid oxidation generally generates a large quantity of such types of energy. However, erythrocytes and adrenal medulla cannot utilize fatty acids for energy requirements.
Anaerobic Conditions
Fermentation is substrate-level phosphorylation of organic compounds in the absence of air. The equation for the substrate-level phosphorylation of glucose to form lactic acid is:
C6H12O6 + 2ADP + 2Pi → 2CH3CH(OH)COOH + 2ATP + 2H2O
During photosynthesis, adenosine triphosphate (ATP) can be produced in plants through a process called photophosphorylation. In plants, such molecules can be synthesized in the thylakoid membrane of the chloroplast.
The majority of adenosine triphosphate or ATP molecules in the human body can be recycled from ADP molecules. Therefore, the total amount of ADP + ATP in the human body is nearly fixed at a given time. However, the hydrolysis of ATP can provide the daily energy used by adult human cells.
Functions of Adenosine Triphosphate
Intracellular Signaling
Adenosine triphosphate (ATP) is a phosphate donner that activates various protein kinases which phosphorylate other proteins in living systems. Phosphorylation of a protein by a kinase can trigger signaling cascades for various cellular processes. Therefore, it serves as a key energy source for signal transduction pathways within the biological cell.
DNA and RNA Synthesis
Adenosine triphosphate (ATP) is one of the most important components that help in the synthesis of DNA and RNA. Adenosine triphosphate is one of four monomers that are required for the biochemical synthesis of RNA by RNA polymerases. Therefore, adenosine from ATP is the basic building block of RNA molecules.
However, during DNA synthesis, the enzyme ribonucleotide reductase can reduce the sugar residue of ribonucleoside diphosphates to form deoxyribonucleoside diphosphates (dADP). Many other biochemical reactions such as DNA replication and DNA transcription also consume adenosine triphosphate molecules.
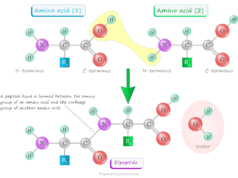
Amino Acid Activation in Protein Synthesis
Amino acids are generally activated and attached to tRNS in a two-step biochemical reaction. A group of enzymes such as aminoacyl tRNA synthetases are required for this activation process. Such enzymes are highly specific for the amino acid and the corresponding tRNA.
In the first step, the amino acid can attached to the enzyme utilizing adenosine triphosphate (ATP) to form an enzyme-AMP-amino acid complex.
Amino acid + ATP ⟶ AMP-amino acid complex + PPi
In the next step, the amino acid can transferred to the 3’ end of the tRNA to form aminoacyl tRNA.
AMP-amino acid complex + tRNA ⟶ Aminoacyl tRNA + AMP
ATP Binding Cassette Transport
ATP binding cassette (ABC) proteins are transmembrane proteins associated with membrane ATPases. They mostly use energy from adenosine triphosphate hydrolysis to transport lipids, vitamins, and drugs in and out of the cells and across the intercellular components.
Hundreds of ABC transporters have been identified and participate in various biological processes in living cells. Defects of adenosine triphosphate or ATP binding cassette (ABC) may cause several disorders such as cystic fibrosis, Tangier disease, respiratory distress syndrome, and macular degeneration.
Extracellular Signalling
Adenosine triphosphate (ATP) is a neurotransmitter that can communicate with other cells in a process of purinergic signaling. Such extracellular signaling is a key mechanism for communication of the nervous system by using purine nucleotides and nucleosides. It commonly activates purinergic receptors in the cell or in nearby cells and regulates the biological functions of cells.
Therefore, it is a critically important signaling molecule for microglia-neuron interactions in the adult brain. Adenosine triphosphate can also modulate ciliary beating and affect vascular oxygen supply.
Muscle Contraction
Adenosine triphosphate (ATP) is an important component for the contraction of muscle. During the relaxation of muscle contraction, the S1 head of myosin hydrolyses ATP to ADP and Pi. It results in the formation of a high-energy ADP-Pi myosin complex.
During contraction, the muscle gets stimulated by the precipitation of actin, calcium ion (Ca+2), troponin, and tropomyosin to form the actin-ADP-Pi complex. The next step is the power stroke which drives the movements of actin filaments over myosin filaments. It is followed by the release of ADP and Pi. It also changes the conformation of myosin and makes the actin-myosin complex in a low-energy state.
A fresh adenosine triphosphate molecule now binds to form an actin-myosin ATP complex. Actin is released because myosin ATP has a low affinity for actin. It is a crucial step for relaxation and depends on the binding of ATP to the actin-myosin complex. Therefore, adenosine triphosphate in muscle contraction and relaxation cycle is the source of energy.
Frequently Asked Questions
What is ATP?
Adenosine triphosphate (ATP) in biology is a unique and most important high-energy biomolecule in living cells. Therefore, adenosine triphosphate is an energy currency of the cell in living organisms that control various biological processes.
Why is ATP important?
Adenosine triphosphate is the main energy currency of the cell in living organisms. Therefore, adenosine triphosphate in biology is a unique and most important high-energy biomolecule in living cells because such energy is used by the cell in several metabolic processes as well as in the synthesis of biomolecules such as proteins.
How does ATP release energy?
A huge amount of energy can be released during the enzymatic removal of one phosphate group from ATP to form ADP.
ATP + H2O → ADP + Pi + 7.3 Cal (30.5 kJ)
The removal of a second phosphate group from ATP also releases energy to form adenosine monophosphate (AMP).
ATP + H2O → AMP + PPi + 10.9 kcal (45.6 kJ)
AMP and ADP can converted to ATP when energy is not needed by the living cells. Therefore, adenosine triphosphate is a reliable energy source for various biological processes in living cells.
What is ATP used for in cells?
Adenosine triphosphate is the main source of energy that is used by cells to perform various biological processes. Due to the presence of unstable high-energy bonds, adenosine triphosphate can readily hydrolyze to emit a large amount of energy. Such energy currency is used by the cell in several metabolic processes as well as in the synthesis of biomolecules such as proteins.