Potassium Chloride Salt
Potassium chloride (chemical formula KCl) is a metal salt of potassium and chlorine atom. It is a mineral supplement used mainly in medicine to treat or prevent low blood potassium levels or hypokalemia. A major part of potassium chloride is used in agriculture for making fertilizers like Muriate of Potash or MOP. The colorless crystalline solid form of potassium chloride salt can be easily dissolved in water to form a salty KCl solution. Potassium chloride salt is formed by ionic bonding between a potassium atom and a chlorine atom. It is made up of potassium cations and chloride anions in a 1:1 ratio.
Chemistry
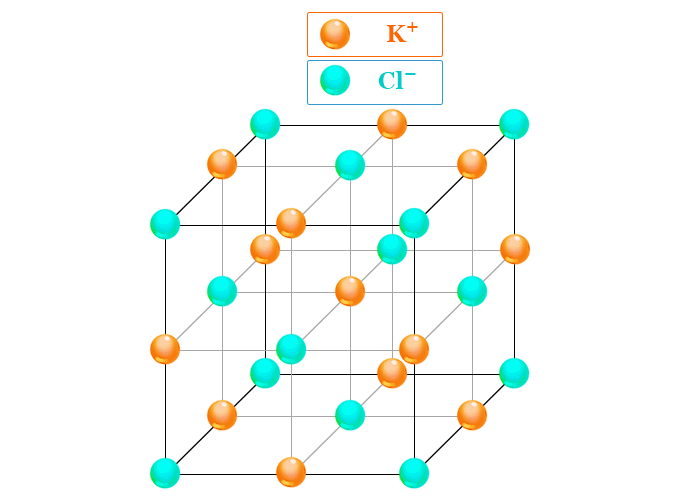
The crystals of potassium chloride salt are similar to that of sodium chloride. Both the salts adopt a face-centered cubic crystal structure.
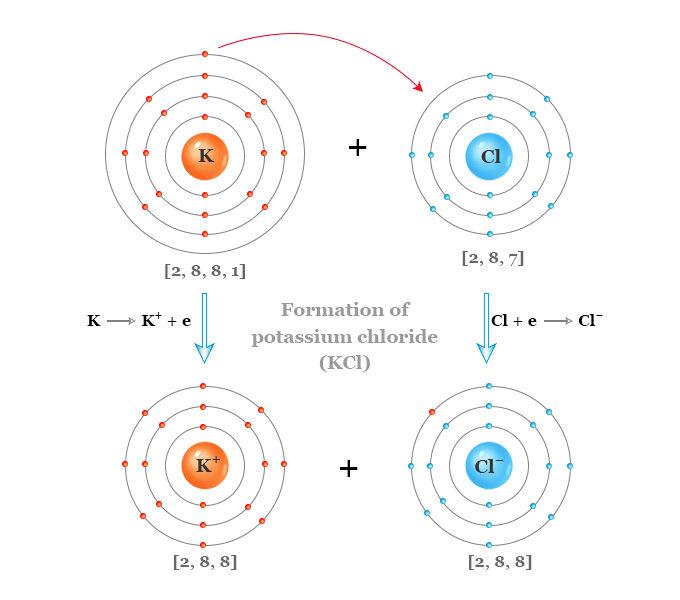
Each potassium atom loses one electron to form a unipositive potassium ion (K+). Similarly, the chlorine atom gains one electron to form a uni negative Cl− ion. The two oppositely ions K+ and Cl− are held together by the electrostatic force of attraction to form crystalline KCl salt.
Properties
All alkali metal halides are colorless crystalline solids with high melting and boiling points. They are highly soluble in water. The common properties of KCl are given below in the table,
| Name | Potassium chloride |
| Other names | Sylvite Muriate of potash or MOP |
| Chemical formula | KCl |
| Molar mass | 74.555 g mol−1 |
| Appearance | White crystalline solid |
| CAS Number | 7447-40-7 |
| Density | 1.984 g/cm3 |
| Melting point | 770 °C |
| Boiling point | 1,420 °C |
| Solubility | Soluble in water, liquid ammonia, liquid sulfur dioxide, methanol, formic acid, acetone, acetamide, etc. |
| Acidity (pKa) | ≈ 7 |
| Crystal structure | face-centered cubic crystal lattice |
Chemical properties
- It is completely ionized in water to form K+ and Cl− ions. Therefore, an aqueous solution of KCl shows a high value of electrical conductivity.
- The pH of an aqueous solution of KCl ≈ 7 because the aqueous solution of KCl forms a strong acid (HCl) and a strong base (KOH).
- Commercial production of potassium by electrolysis of molten KCl is not favorable because potassium is quite soluble in the molten electrolyte KCl. Therefore, the commercial method involved the reduction of molten KCl by sodium metal at 850 °C.
KCl + Na → NaCl + K
The reduction is rather unusual because potassium is a better reducing agent than sodium. However, the higher volatility of potassium in the temperature range of 850 °C to 880 °C shifts the equilibrium toward the right.
Preparation of potassium chloride
Potassium chloride is an important constituent for the production of potassium fertilizer (potash). Canada, Russia, and Belarus are the largest producers of agricultural and industrial grade potash fertilizer. It may be prepared by following industrial and laboratory processes,
- KCl is extracted from natural minerals such as sylvite, carnallite, and potash.
- It is also obtained as a by-product during the synthesis of nitric acid from hydrochloric acid and potassium nitrate.
KNO3 + HCl → HNO3 + KCl - It is inexpensively available but rarely prepared in the laboratory. It is prepared in the laboratory by treating potassium hydroxide with hydrochloric acid.
KOH + HCl → KCl + H2O - It is also obtained in the laboratory by burning potassium in the presence of chlorine gas. The process is highly explosive.
2 K + Cl2 → 2 KCl
Potassium chloride uses
In everyday life, potassium chloride salt uses mainly as a medicine and as a fertilizer. It is also used in chemical industries and water softening processes.
Uses in fertilizer
The majority of the potassium chloride produced in the world is used to make potassium fertilizer (potash) due to its low cost and it enriches more K in soil than other sources. It promotes the growth of plant life.
The fertilizer made from KCl is called Muriate of Potash or MOP. It rapidly dissolves in soil water and K+ will be retained on the negatively charged cation exchange sites of clay and organic matter. The Cl− part is readily removed by water. Therefore, it increases the concentration of potassium in the soil.
It may also be applied to the seed. Since dissolving fertilizer will increase the soluble salt concentration near the seed to avoid damaging the germination of plants.
Potassium chloride in water softener
Potassium chloride is a naturally occurring essential nutrient found in various fruits, vegetables, meat, and dairy products. In water softening units, we use KCl as an alternative to sodium chloride. Using potassium chloride crystals in a water softener system can replace magnesium and calcium from hard water with potassium.
Other uses
- In the chemical industry, KCl is used to manufacture potassium hydroxide and potassium metal.
- Glass Manufacturers Company uses granular potash for the production of eyeglasses, glassware, televisions, and computer monitors. Using KCl in glass manufacture imparts excellent clarity to the glass.
- It is also used as a substitute for common salt or sodium chloride in food products.
- It is used as a fire extinguishing agent in portable and wheeled fire extinguishers.
- KCl is useful as a beta radiation source in the calibration of radiation detection instruments.
- Along with sodium chloride and lithium chloride, the KCl flux is used for the gas welding of aluminum.
Potassium chloride medication
Potassium is an important mineral for the human body. The deficiency of potassium in our body causes various health problems. Therefore, we use potassium chloride tablets or intravenous injections to maintain our low blood potassium levels. According to the World Health Organization, it is an essential medicine for human health.
Potassium is a mineral that is needed for the proper functioning of the heart, muscles, kidneys, nerves, and digestive system. Certain diseases, illnesses, and drugs can decrease the potassium level in the human body. Therefore, we need KCl supplements to maintain lost potassium levels.
Potassium chloride supplement in animals is used to boost the potassium level in the feed. It is also beneficial in milk production.
Side effects of potassium chloride
Potassium chloride is beneficial for human health but a high level of KCl causes various types of side effects. It commonly causes stomach pain, nausea, vomiting, discomfort, or diarrhea.
The serious side effects of KCl may include uneven heartbeat, muscle weakness or limp feeling, stomach pain, and tingling in your hands, feet, or mouth. Therefore, always ask your doctor before taking potassium chloride supplements like the oral solution or tablet. You should not use KCl medication if you have high levels of potassium in your blood or hyperkalemia.
Potassium chloride solution
KCl is an ionic compound. Therefore, it may be soluble in a variety of solvents. In water, it readily dissolved to form a potassium chloride solution.
| Temperature (°C) | Solubility in g per 100 g of water |
| 0 | 28.15 |
| 20 | 34.24 |
| 40 | 40.3 |
| 60 | 45.6 |
| 80 | 51 |
| 100 | 56.2 |
KCl is also soluble in many other solvents like liquid ammonia, liquid sulfur dioxide, methanol, formic acid, acetone, acetamide, etc.
Potassium chloride in food
Potatoes and bananas are naturally occurring potassium-rich foods which contain potassium in the form of potassium citrate. Some other processed foods and supplements contain potassium in the form of potassium chloride. Both these sources help to lower your blood pressure and increase the potassium levels in your blood. The common food supplements which contain KCl may include
- Dairy and egg products: Potassium chloride acts as a thickener, stabilizer, firming agent, and flavor enhancer in many dairy and egg products such as chocolate milk, condensed milk, powdered milk, yogurt, pudding, salted eggs, etc.
- Grains and cereals: The most common potassium reached grains and cereals may include brown and wild rice, bran cereal, whole-wheat bread, pasta, etc.
- Fruits and vegetables: Vegetables like cooked spinach, cooked broccoli, potatoes, sweet potatoes, peas, cucumbers pumpkins, and leafy greens contain high levels of potassium. Many fruit juices like orange juice, tomato juice, prune juice, apricot juice, and grapefruit juice are also helpful to increase your blood potassium level.
- Protein-rich foods: Protein-rich foods like nuts, canned or fermented seafood, processed meat or poultry, and soybeans contain a high level of potassium chloride salt.