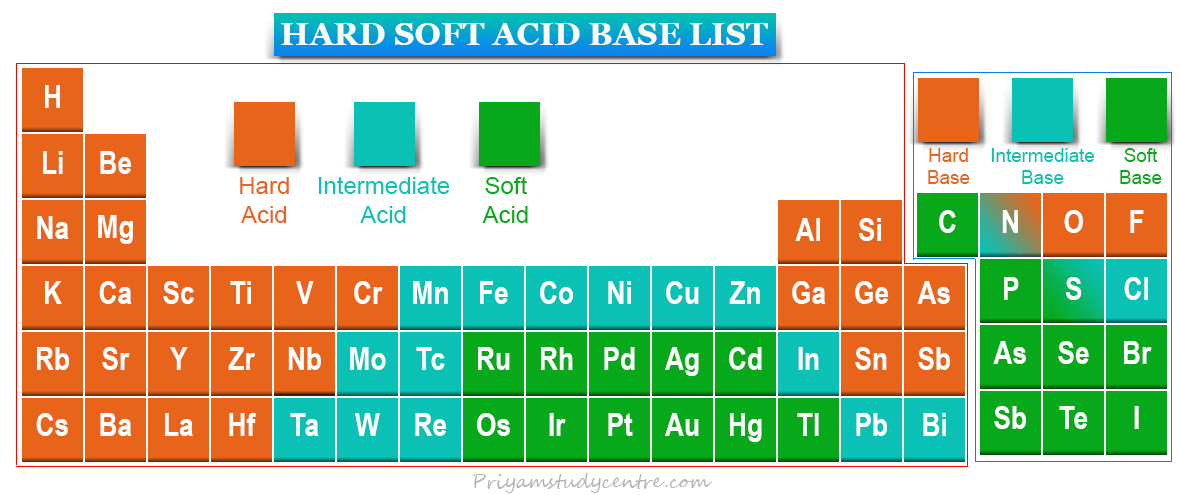
Hard Soft Acid Base List
Hard soft acid base theory or HSAB principle was proposed by Ralph Pearson in 1963. HSAB principle proposed that the chemical complex is most stable when participating acids and bases are either both soft or hard. However, Lewis theory explains the list of acid-base neutralization reactions of hard or soft acids and bases in terms of electronic configuration with the formation of the complex by a coordinate covalent bond between the vacant orbital of an acid and the filled orbital of a base. Experimental chemical reactions of acids and bases indicate that hard acid prefers to combine with hard bases and soft acid prefers to bind with soft bases. It is very helpful for predicting the stability of acids bases in a chemical complex.
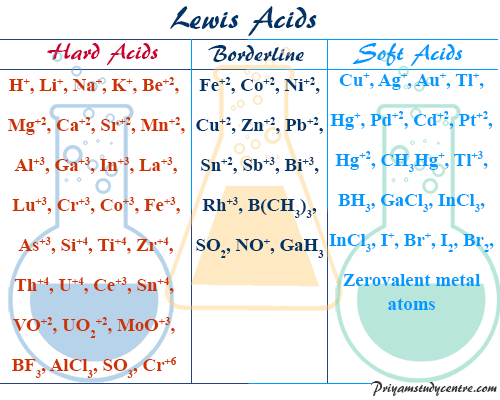
The hard-soft acid-base (HSAB) principle explains the stability of these acid-base complexes. Soft acids form stable complexes with bases that are high in electric polarization and good reducing agents. Therefore, the hard acid and the hard base will equilibrium to form a stable complex.
A (acceptor) + :B (donor) → A : B (adduct or complex)
Hard Acid and Soft Acid
In simple terms, hardness is associated with a tightly held electron with little tendency to polarity. However, softness is associated with a loosely bound polarizable electron.
Hard Acids
Hard acids have small acceptor atoms, high positive charge, and do not contain unshared pairs of electrons in their valence shell. All these properties may not appear in the same acids. Therefore, these basic properties lead to high electronegativity, low polarization, and hard to oxidize.
For example,
- Nitrogen (N) > Phosphorus (P)
- Oxygen (O) > S (Sulfur)
- Fluorine (F) > Chlorine (Cl) > Bromine (Br) > Iodine (I)
Among these chemical elements, the electronegativity and ionization energy of nitrogen is greater than phosphorus thus hardness of nitrogen is greater than phosphorus. Similarly, the electronegativity of oxygen is greater than sulfur. Therefore, the hardness of oxygen is greater than sulfur. In the same way, we can explain the hardness of halogens.
Soft Acids
Soft acids have large acceptor atoms, low positive charge, and contain ushered pairs of electrons in their valence shell. All these properties may not appear in the same acid. This definition of acid leads to high polarity, low electronegativity, and electron affinity.
The softness of periodic table chemical elements,
- Nitrogen (N) < Phosphorus (P)
- Oxygen (O) < Sulfur (S)
- Fluorine (F) < Chlorin (Cl) < Bromine (Br) < Iodine (I)
Among these chemical elements, electronegativity and electron affinity of oxygen are greater than sulfur thus softness of oxygen is lower than sulfur.
Hard and Soft Base
Hard bases are those in which the donner atoms have low polarizabilities and high electronegativities.
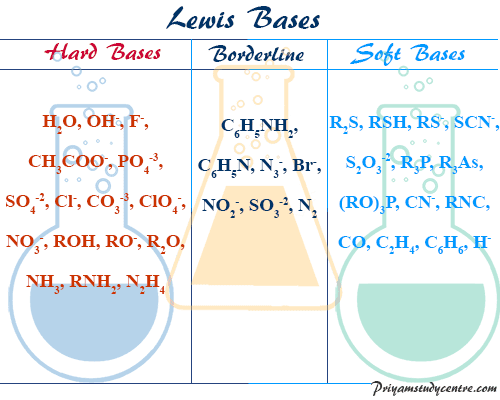
Soft bases are those in which the donner atoms have high polarizabilities and low electronegativities.
Hard Soft Acid Base Examples
- In simple terms, the hardness of bases is associated with a tightly held electron with little tendency to polarity.
- On the other hand, the softness of bases is associated with a loosely bound polarizable electron.
Within the group of the periodic table with the increasing atomic number, the softness of the Lewis bases increases. Among the halide ions, softness increases from top to bottom in the periodic table,
Fluoride (F−) <chloride (Cl−) < bromide (Br−) < iodide (I−)
From the above, F− is the hardest, and I− is the softest Lewis base.
Question: Which of the following chemical compounds are soft and hard acids and bases,
Answer:
- The hydride ion has a negative charge and is too large in size compared to the hydrogen atom. Hence the electronegativity of hydride ions is quite low and polarizability is very high. So the valence electron in the hydride ion is loosely bound by the nucleus and it is a soft base.
- Nickel (IV) has quite a high nuclear charge and a small size compared to nickel (II). Hence electronegativity of nickel (IV) will be very high and polarizability will be low. Therefore, nickel (IV) is a hard acid.
- Mono-positive iodine has a low positive charge and has a large size. Therefore, the low electronegativity and high polarizability form a soft base.
- Hydrogen ion has the smallest dimensions with a high positive charge density and absence of the unshared pair of electrons in its valence shell. All these will give a high electronegativity and very low polarizability. Hence ionic hydrogen bonding is easy to a hard base and acts as a hard acid.
HSAB Theory
This principle was proposed in 1963 by Ralph G. Pearson, according to the hard soft acid base or HSAB principle, the chemical complex is most stable when participating acids and bases are either both soft or hard. Hydrogen ion and methyl mercury (II) ion are used for this comparison in inorganic chemistry.
HSAB Theory Examples
Hard or soft acid like hydrogen ions and methyl mercury ions has only one coordination position to form only one coordinated chemical bond. However, these two cations are different in their preference for bases. The preference was estimated from the experimental determination of chemical equilibrium constants for the redox reaction.
BH+ + [CH3Hg(H2O)]+ → [CH3HgB]+ + H3O+
The experimental results indicate that the bases in which nitrogen, oxygen, or fluorine is the donor atom, prefer to coordinate with the hydrogen ion.
But the bases in which phosphorus, sulfur, iodine, bromine, chlorine, or carbon are donor atoms, prefer to coordinate with mercury.
Difference Between Hard Acid and Soft Acid
- Hard acids have small acceptor atoms and a positive charge while the bases have small donor atoms but often with a negative charge. Hence a strong ionic interaction will lead to the hard acid-base combination.
- On the other hand, a soft acid-base combination is mainly a covalent interaction. Soft acids have large acceptor atoms, low positive charge, and contain ushered pairs of electrons in their valence shell.
Application of HSAB Principle
The hard soft acid base or HSAB principle is extremely useful in elucidating many useful properties of learning chemistry.
Why the behavior of boron in boron trifluoride and boron trihydride is different?
Due to the presence of hard fluoride ions in boron trifluoride easy to add hard bases. However, the presence of soft hydride ions in boron trihydride easy to add soft bases.
Metal Ore and Hard Soft Acids Bases
The concentration of certain ore on the earth’s surface can be rationalized by applying the hard-soft acid-base or HSAB principle.
- According to the hard soft acid base principle, magnesium, calcium, and aluminum occur on the earth’s surface as magnesium carbonate, calcium carbonate, and aluminum oxide but magnesium calcium and aluminum sulfides did not occur in the earth’s environment.
- On the other hand, soft acids such as Cu+, Ag+, and Hg+2, occur in nature as sulfides. The borderline acids such as Ni+2, Cu+2, and Pb+2 occur in nature both as carbonates and sulfides.
The definition and chemical properties of the hard-soft acid-base combination list provide the occurrence of the complex ore in our environment.
Hard acids and bases are stable through ionic bonding and soft acids and bases are stable through covalent bonding. For example, the crystalline solid molecule of Mg(OH)2 forms through ionic bonding while the HgI2 molecule through covalent chemical bonding by Hard soft acid-base reaction.
Hard Soft Acid Base Problems
Why [CoF6]−3 is more stable than [CoI6]−3?
In [CoF6]−3 and [CoI6]−3 both complexes of cobalt have a +3 oxidation number or state and act as a hard acid. But fluoride ion is a hard base and iodide ion is a soft base.
Therefore, in [CoF6]−3, the hard acid and hard base form a more stable complex than the [CoI6]−3 form by a hard acid and soft base.
Why silver iodide is stable but silver fluoride does not exist?
We know that mono-positive silver ion is a soft acid. But fluoride ion is a hard base and iodide ion is a soft base.
Therefore, from the HSAB principle, soft acid silver will prefer to combine with the soft base iodide ion. Hence silver iodide is stable but silver fluoride does not exist.
Why is a hard-soft acid-base complex like mercury hydroxide more readily dissolved in low pH solution than mercury sulfide?
Mercury is a soft acid, and hydroxide and sulfide are a hard base and soft base respectively. Thus mercury sulfide formed by soft acid-base will be more stable than mercury hydroxide formed by soft acid and hard base.
Therefore, due to lower stability mercury hydroxide dissolved readily in a low pH solution but mercury sulfide does not readily dissolve in a low pH or acidic solution.