Conjugate Acid Base Pair Definition
Conjugate acid base pair or protonic definition of acids and bases was independently proposed by Bronsted and Lowery in 1923. According to this definition, an acid is a proton donor and a base is a proton acceptor in chemistry. Conjugate acid-base theory can not explain the conjugate acid base phenomena in terms of electronic structure by the formation of the coordinate covalent bond between the vacant orbital and the orbital which contains a lone pair of electrons or Lewis acid base theory.
For learning chemistry, the conjugated acid base theory may be defined broadly as,
- A conjugate acid is any hydrogen containing material (molecule or ion) that can release a proton or hydrogen ion to any other substances.
- A conjugate base is any substance (molecules or ions) that can accept a proton from any other substances to form the conjugate acid base pair.
Conjugate Acid Base Pair List
The neutralization reaction going through donates or accepts protons by acids and bases. Hence acids base pairs are the members of the balanced chemical equation that can be formed by mutual loss or gain of protons.
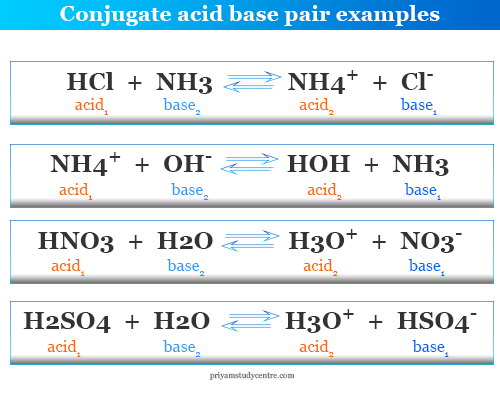
Conjugate Acid Base Pair Examples
For example, nitric and sulfuric acid easily donate a proton to water to show acid character. Water easily accepts a proton from sulfuric or nitric acid to show base properties.
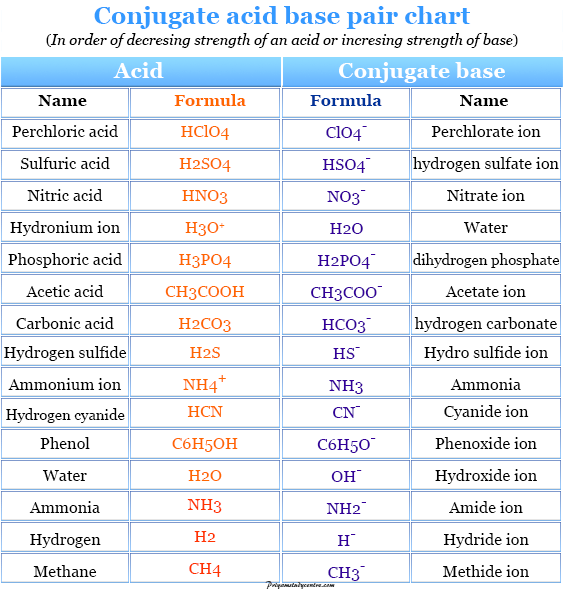
In the below list, we provide some examples of conjugated acid-base pairs with their name and formula.
| Conjugate acid | Conjugate Base | ||
| Name (in order of increasing strength) | Formula | Name (in order of decreasing strength) | Formula |
| Perchloric acid | HClO4 | Perchlorate ion | ClO4− |
| Sulfuric acid | H2SO4 | Hydrogen sulfate ion | HSO4− |
| Hydrogen chloride | HCl | Chloride ion | Cl− |
| Nitric acid | HNO3 | Nitrate ion | NO3− |
| Hydronium ion | H3O+ | Water | H2O |
| Hydrogen sulfate ion | HSO4− | Sulfate ion | SO4−2 |
| Phosphoric acid | H3PO4 | Dihydrogen phosphate ion | H2PO4− |
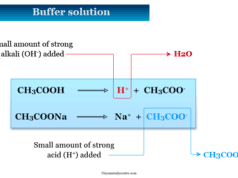
| Acetic acid | CH3COOH | Acetate ion | CH3COO− |
| Carbonic acid | H2CO3 | Hydrogen carbonate ion | HCO3− |
| Hydrogen sulfide | H2S | Hydro sulfide ion | HS− |
| Ammonium ion | NH4+ | Ammonia | NH3 |
| Hydrogen cyanide | HCN | Cyanide ion | CN− |
| Hydrogen carbonate ion | HCO3− | Carbonate ion | CO3− |
| Phenol | C6H5OH | Phenoxide ion | C6H5O− |
| Water | H2O | Hydroxide ion | OH− |
| Ethyl alcohol | C2H5OH | Ethoxide ion | C2H5O− |
| Ammonia | NH3 | Amide ion | NH2− |
| Methyl amine | CH3NH2 | Methyl amide ion | CH3NH− |
| Hydrogen | H2 | Hydride ion | H− |
| Methane | CH4 | Methide ion | CH3− |
How do you find the conjugate acid base pair?
When an acid release a proton, the residue must be a base and this can take up a proton to form the original one. The neutralization reaction involved two acids or two bases forming conjugated acids and base pairs.
It defines acids and bases in terms of the substances themselves and not in terms of the pH scale of the acid base solution.
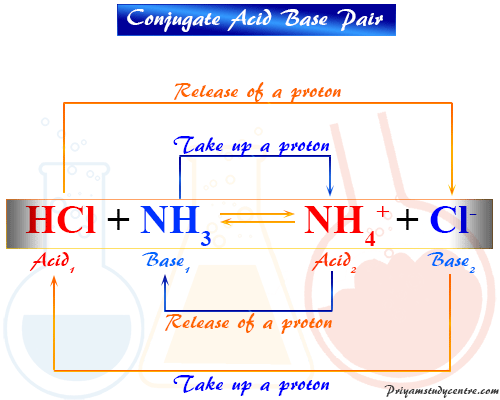
HCl + H2O → H3O+ + Cl−
In this reaction, proton plays a key role in acid base function. Hydrogen ion has a very high charge density and is effective for the electric polarization of other ions or molecules according to Fajan’s rules. Water accepts a proton from hydrochloric acid. Therefore, water is a conjugate base of HCl.
In the reverse reaction which at chemical equilibrium proceeds at the same rate as the forward reaction. Here H3O+ ion donates a proton to Cl− ion or Cl− ion accepts a proton from H3O+ ion. Therefore, HCl is the conjugate acid of water.
Which of the following is a conjugate acid-base pair?
Conjugate acid base pairs are formed from each other mutually by the gain or loss of protons. The equilibrium reaction involves two acids and two bases.
The stronger acid and weaker base form one conjugate pair and the stronger base and weaker acid form another pair.
Some common examples for formation conjugate acids and bases are,
HClO4 ⇆ H+ + ClO4−
H2SO4 ⇆ H+ + HSO4−
HCl ⇆ H+ + Cl−
HNO3 ⇆ H+ + NO3−
H3O+ ⇆ H+ + H2O
On the basis of Bronsted Lewary theory or protonic definition, the neutralization reaction involves acid1 and base1 forming one pair, and acid2 and base2 forming another conjugate acid base pair.
Conjugate Base of Weak Acid
Methane, hydrogen peroxide, and hydrogen molecule are the weakest acids, and their conjugate bases are CH3−, H2O, and H−. These are consequently the strongest bases.
Weak acid produces a strong conjugate base and strong acid produces a weak conjugate base. If we consider hydracids of the 2nd period in the periodic table like methane (greenhouse gas), ammonia, water, and hydrogen fluoride.
Polarity and acidity increase from methane to hydrogen fluoride. But after donating protons these acid molecules form conjugate bases. The basicity decreases from NH2− to F− ion. Therefore, the fluorine ion (F−) is a weak base, and NH2− ion is a strong base.
Conjugate Base Strength
According to Bronsted theory, a strong acid has a strong affinity to donate the proton but a strong base has a strong affinity to accept the proton.
When we compare the strength of the conjugate base of hydrogen cyanide and acetic acid, the experimentally observed ionization or acidity constant at 25°C,
KCH3COOH = 1.8 × 10−5 and
KHCN = 4.0 × 10−10
Therefore, the acidity of acetic acid is greater than that of hydrogen cyanide.
The second method is comparative pyrolysis or thermal decomposition by specific heat. In this method, we determined the equilibrium concentration of the conjugate acid base.
For example, ethoxide ions fairly chemical bonding with water to form ethyl alcohol than hydroxyl ions. Therefore, ethoxide ion is a stronger base than hydroxyl ion, and water is a stronger acid than ethyl alcohol.
Acid Base and Oxidation Number
The acidic character of the conjugate acid base pair of oxyacids of the same chemical element increases with the increasing oxidation number.
For example, the oxyanions of a series of chlorine oxoacids HOCl, HClO2, HClO3, and HClO4 are ClO−, ClO2−1, ClO3−1, and ClO4− respectively. All these four acids have one protonated oxygen atom.
- The acidic character of the oxoacids increases from HClO to HClO4 because the oxidation number of the central atom or chlorine increases from HClO to HClO4.
- The basic character of the conjugate base or oxyanions decreases from ClO− to ClO4−.
If we consider the conjugate acid base pair of the oxoacids of phosphorus this rule is not applied due to the structure of these oxoacids.