What is Acid Base Titration?
Acid base titration in chemistry is an experimental procedure used to calculate the unknown concentration of an acid or base by an indicator. When the strong acid or base is titrated in the presence of a weaker one, the endpoint of the acid base titration is much sharper. Many inorganic and organic compounds can be determined in the laboratory by an acid base titration based on their acidic or basic properties.
If the pH of the equivalence point is 4 to 10, acid base titration gives a sharp endpoint.
Types of Acid Base Titrations
The acid base titrations are classified into four different types such as strong acid-strong base, weak acid-strong base, strong acid-weak base, and weak acid-weak base.
We commonly use the following strong and weak acids and bases to perform four different types of acid base titration:
- Strong acids: Hydrochloric acid (HCl) and sulphuric acid (H2SO4)
- Weak acids: Acetic acid (CH3COOH) and formic acid (CH2O2)
- Strong bases: Sodium hydroxide (NaOH) and potassium hydroxide (KOH)
- Weak bases: Ammonia (NH3) and methylamine (CH3NH2)
Strong acid-strong base
In a strong acid-strong base titration, the acid and base will combine to form a neutral solution. Typically, we use sodium hydroxide as a strong base and hydrochloric acid as a strong acid to perform this titration process.
NaOH (aq) + HCl (aq) ⇌ NaCl (aq) + H2O (l)
At the equivalence point in this titration, hydronium (H3O+) and hydroxide (OH−) ions will react to form water.
H3O+ + OH− ⇌ 2H2O
It led to achieving a pH of 7.
Weak acid-strong base
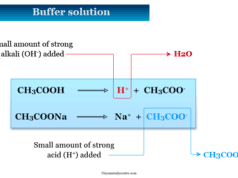
The titration of a weak acid such as acetic acid or ethanoic acid with a strong base such as sodium hydroxide involves the direct transfer of protons from the weak acid to the hydroxide ion.
CH3COOH (aq) + OH− (aq) ⇌ CH3COO− (aq) + H2O (l)
In this reaction, the acid and base react in a one to one ratio. At the equivalence point of the titration, the pH value is greater than 7 because all of the acid (HA) has been converted to its conjugate base (A−) by the addition of sodium hydroxide (NaOH).
Therefore, the equilibrium moves backward towards HA and produces hydroxide ions in the solution.
A− + H2O ⇌ HA + OH−
Strong acid-weak base
In a strong acid-weak base titration, the acid and base will react to form an acidic solution with a pH lower than 7. We use ammonia as a weak base and hydrochloric acid as a strong acid to perform this titration process.
HCl (aq) + NH3 (aq) ⇌ NH4Cl (aq)
A conjugate acid will be produced during the titration of strong acid and weak alkali. It then reacts with water to form hydronium ions in the solution.
Weak acid-weak base
There are no sharp changes in the titration curve of a weak acid and weak base titration process. Therefore, no suitable indicator is present to carry out such a titration process.
We commonly use ammonia as a weak base and ethanoic acid as a weak acid to perform this titration process.
CH3COOH (aq) + NH3 (aq) ⇌ CH3COONH4 (aq)
In this titration, the pH gradually changes around the equivalence point. It is greater or less than 7.
Principle of Acid Base Titration
An acid-base titration principle is used to determine various properties of strong or weak acids or bases. They can be used to determine the following properties.
- To determine the concentration of unknown organic and inorganic acids or bases.
- To determine the pH value of an unknown acid or base.
- Acid strength (pKa) of an unknown acid or alkalinity (pKb) of the unknown base.
Acid base titrations in analytical chemistry work on the principle that the number of equivalents of unknown concentrations of an acid or base should be equal to the number of equivalents of known concentrations of an acid or base.
In a neutralization process, an acid reacts with a base to form salt and water.
Acid + Base ⇌ Salt + Water
Therefore, in acid base titrations, the strength of an acid of unknown concentration can be estimated by reacting it with a suitable base of known concentration.
We use V1N1 = V2N2 to calculate the concentration or strength of an unknown acid or base.
Where V1 is the volume of the titrant used in the titration
N1 is the normality of the titrant
V2 is the volume of the analyte
N2 is the normality of the analyte
Practice Problems
Problem: Calculate the normality of 50 mL of KOH when it is titrated against 100 mL of 0.2 M H2SO4.
Solution: According to the law of equivalence, in a neutralization reaction, V1N1 = V2N2
Volume of sulfuric acid (V1) = 100 mL
Volume of pottasium hydroxide (V2) = 50 mL
Normality of H2SO4 (N1) = acidity × molarity
= 2 × 0.2 = 0.4
Let the normality of KOH = x
From the above formula, 100 × 0.4 = 50 × x
or, x = 0.8
Therefore, the normality of 50 mL of KOH is 0.8
Problem: Calculate the molarity of 75 mL of barium hydroxide when it is titrated against 150 mL of 0.3 N hydrochloric acid.
Solution: According to the law of equivalence, in a neutralization reaction, V1N1 = V2N2
The volume of hydrochloric acid (V1) = 150 mL
The volume of barium hydroxide (V2) = 75 mL
Normality hydrochloric acid (N1) = 0.3
Let the normality of Ba(OH)2 = x
From the above formula, 150 × 0.3 = 75 × x
or, x = 0.6
Therefore, the molarity of 75 mL of barium hydroxide is normality/basicity = 0.6/2 = 0.3.
Important Terms in Acid Base Titration
Analyte
The analyte is a substance whose concentration can be determined in acid base titrations.
Titrant
In analytical chemistry, a titrant is the solution of a known concentration that is added to the analyte in a titration process. The titrant may also be named as titrator, the reagent, or the standard solution that use to determine the concentration of an analyte.
Endpoint
The point at which the chemical reactions between the analyte and the titrant are balanced is called the endpoint in acid base titration. It is usually described by a change in the colour, electrical conductivity, or a change in the pH of the solution.
Equivalence point
The equivalence point of an acid base reaction is a point in a titration at which the amount of titrant added is just enough to complete the neutralization process of the analyte solution.
At the equivalence point in an acid-base titration process, moles of base = moles of acid. Therefore, the solution at the equivalence point only contains salt and water.
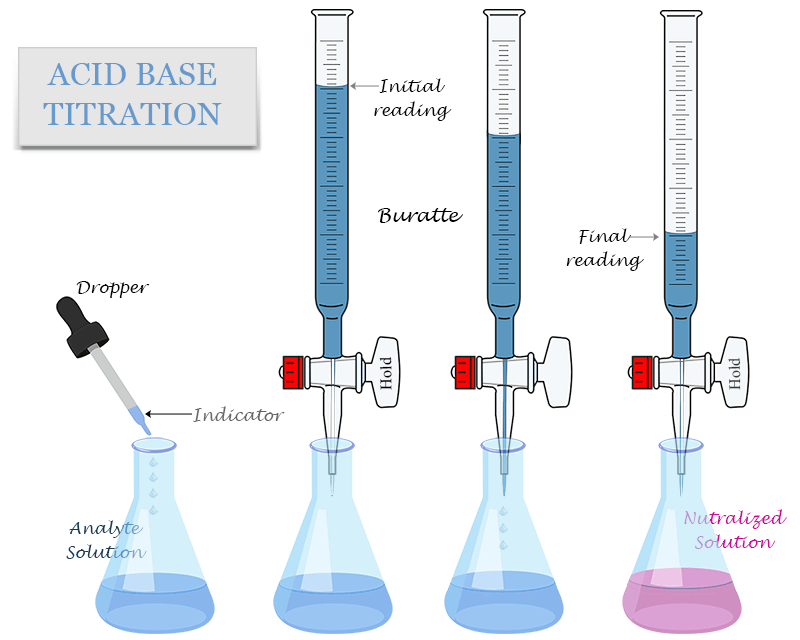
Burette
A burette is a laboratory chemical apparatus, which consists of a long, graduated glass tube, with a stopcock on its lower end.
In various fields of science, it is used to dispense and measure a variable amount of a chemical solution. Burette is used in the laboratory for quantitative chemical analysis to measure the volume of various liquid solutions. The stopcock is a valve that is used to control the flow of the fluid from the burette.
Acid Base Titration Curve
A titration curve is a graphical representation of the pH of a solution during acid base titration.
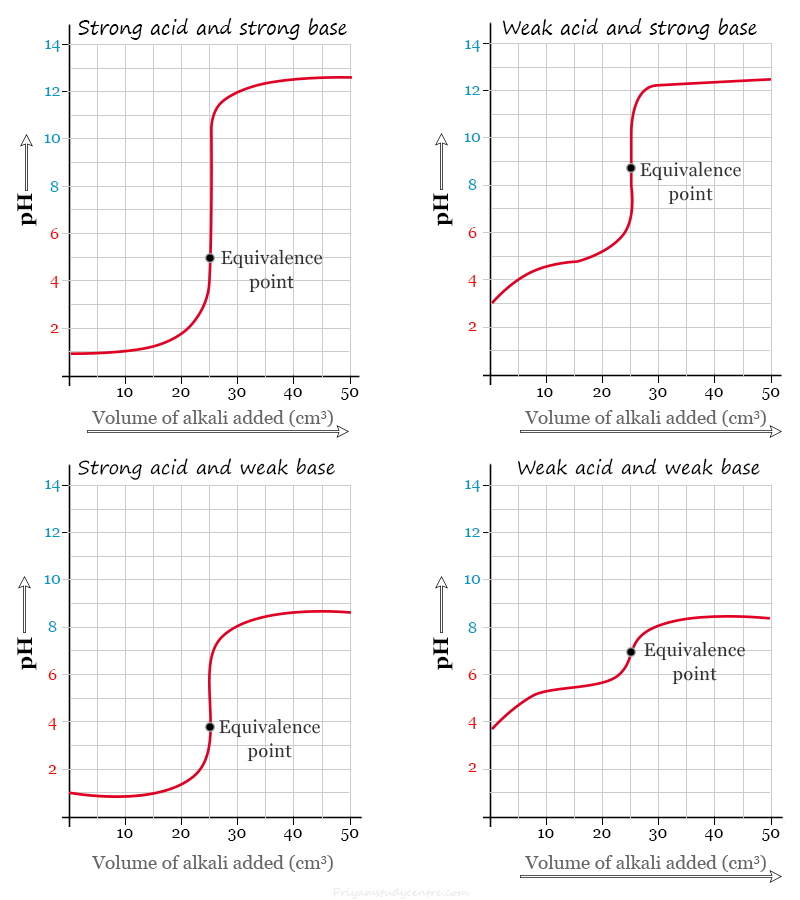
- In a strong acid-strong base titration, the equivalence point or endpoint is reached when the moles of acid and base are equal. The pH of the equivalence point of such titration is 7.
- In a weak acid-strong base titration, the pH value is greater than 7 at the equivalence point.
- For a strong acid-weak base titration, the pH is less than 7 at the equivalence point.
- In a weak acid-weak base titration, the pH gradually changes around the equivalence point.
Acid Base Indicators
Acid base indicators are substances that change their colour or odour when added to an acid or an alkaline solution to indicate the presence of acid or base. In general, acid base indicators are weak organic acids or weak organic bases that show different colours in the ionized and unionized forms.
In aqueous medium acidity is due to the presence of H+ ion. The H+ ion concentration of an aqueous acidic solution can be reduced by the addition of OH− ions. The process of removal of H+ ions by OH− ions or OH− ions by H+ ions is called neutralization.
Such neutralization procedures change the colour of some organic compounds called acid base indicators.
| Indicator | Colour in acid | Colour in alkali | pH range of colour change |
| Methyl orange | Red | Orange | 3.1 to 4.5 |
| Bromo cresol green | Yellow | Blue | 4.0 to 5.6 |
| Methyl red | Red | Yellow | 4.4 to 6.2 |
| Bromothymol blue | Yellow | Blue | 6.2 to 7.6 |
| Phenol red | Yellow | Red | 6.4 to 8.0 |
| Phenolphthalein | Colourless | Red | 8.4 to 10.0 |
| Thymol blue | Colourless | Blue | 9.3 to 10.5 |
| Thymolphthalein | Colourless | Blue | 9.4 to 10.6 |
Importance of Acid Base Titration
Acid-base titrations are important for various fields of science because they help to calculate the unknown concentration of organic or inorganic acids and bases. Therefore, it is a powerful analytical tool in chemistry, biology, environmental chemistry, etc.
- In chemistry or analytical chemistry, acid-base titrations are used to determine the concentration of unknown acids and bases in solutions which are used widely for the preparation of standard solutions. It is also used for understanding acid base reactions.
- In biology or biochemistry, acid-base titrations are used to determine the pH of biochemical substances, which is important for understanding the behavior of enzymes, amino acids, and other biological molecules.
- In environmental chemistry, acid-base titrations are used to determine the acidity or basicity of water, which is important for understanding the impact of acid rain on the environment and aquatic ecosystems.
Overall hundreds of inorganic and organic compounds can be determined in the laboratory by an acid base titration based on their acidic or basic properties.
Process of Acid Base Titration
We need the following apparatus to perform the acid base titration process in the laboratory,
- Conical flask
- Funnel
- Beaker
- Pipette
- Burette
- Burette stand
- Indicator
- Unknown solution
- Standard solution
- Spatula
- Wash bottle
To perform the acid base titration process, we need to follow the following steps,
- First, we arrange the said apparatus to carry out the acid base titration process in the laboratory.
- Then we need to clean all the apparatus with distilled water to perform this titration process.
- After that, we fill the burette with a standardized solution
- Accurately measure the volume of the analyte in the conical flask and add a few drops of indicator in the analyte solution by a dropper.
- Titrate the analyte solution with the standardized solution until the indicator changes its colour. When the indicator permanently changes its color, the equivalence point or endpoint reaches.
- Repeat the acid base titration process at least three more times and record the initial and final readings in the observation table to calculate the concentration of the analyte solution.
Frequently Asked Questions – FAQs
Why is acid base titration important?
Acid-base titrations are an important process in various fields of science because they help to calculate the unknown concentration of an acid or a base in a solution.
Acid base titration is a powerful tool in a variety of fields of science such as chemistry, biology, and environmental chemistry to determine the concentration of unknown acidic or basic samples.
How do acid base titrations work?
Titration is an analytical method used to determine the concentration of an inorganic or organic substance in a solution.
Acid base titration performs when adding a known concentration of an acid or bases (titrant) to an unknown volume of the acid or base (analyte) to measure the volume of the titrant at the endpoint of titrations.
What are the factors that affect acid-base titrations?
The most common factors which affect acid-base titrations are
- The concentration of acid and base
- The volume of acid and base
- Type of indicator used
What is the colour of the universal indicator when the same concentration of 10 mL of H2SO4 is mixed with 10 mL of Mg(OH)2?
The colour of the resulting solution of 10 mL of H2SO4 and 10 mL of Mg(OH)2 with universal indicator is green.
Sulfuric acid (H2SO4) is a strong acid and magnesium hydroxide Mg(OH)2 is a strong base. Therefore, they produce a neutral salt which shows green colour on the universal indicator.
Why does the colour of the indicator change at the endpoint of acid base titrations?
In general, indicators are weak organic acids or weak organic bases that show different colours in the ionized and unionized forms.
Phenolphthalein is a useful indicator because it changes colour between the pH ranges of 8.3-10. In basic solutions, it shows pink colour, and clear in acidic solutions. Therefore, the phenolphthalein indicator is a good choice to perform a weak acid strong base titration.