Ammonia (NH3)
Ammonia (chemical formula NH3) is a colourless pungent smelling gas and highly soluble liquid in water owing to extensive hydrogen bonding. It is a useful refrigerant and solvent that is used widely for the production of fertilizer. The dielectric constant of ammonia is considerably lower than that of water. Therefore, it is a poorer solvent for ionic molecules but better for organic compounds. The effect of strong hydrogen bonding in NH3 reflects its relatively high melting and boiling points. Ammonia is an important source of growing nitrogen in the soil to produce food for the ever-increasing population of the world. Due to the presence of a lone pair in the nitrogen atom, the structure of the ammonia molecule is destroyed tetrahedral or trigonal bipyramidal shape. The H-N-H bond angle in the NH3 molecule is 107.5°.
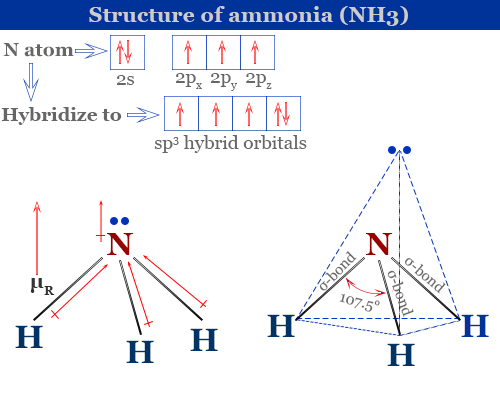
Structure of Ammonia
In an ammonia molecule, the nitrogen atom is the central atom whose valence shell electronic configuration in the ground state is 1s2 2s2 2px1 2py1 2pz1. All four atomic orbitals of nitrogen atom hybridized to form four equivalent sp3 tetrahedral hybridized orbitals.
Three of these four hydridized orbitals contain singly occupied electrons. Therefore, these three sp3 hybrid orbitals overlap with the singly filled 1s orbital of three hydrogen atoms to form three N-H sigma bonds. However, the fourth hybrid orbital contains a lone pair that does not participate in chemical bonding.
Properties of Ammonia
An aqueous solution of ammonia is weakly basic which dissolves to form NH4+ and OH– ions. Therefore, the undissociated ammonium hydroxide molecule (NH4OH) is not known to exist in such solutions.
NH3 (aq) + H2O → NH4+ (aq) + OH– (aq)
| Properties of ammonia |
||
| Chemical formula | NH3 | |
| Molar mass | 17.031 g/mol | |
| Appearance | colourless gas | |
| Odor | pungent smelling gas | |
| Density | 0.769 kg/m3 at STP | |
| Melting point | -77.73 °C | |
| Boiling point | -33.34 °C | |
| Critical temperature | 132.4 °C | |
| Acidity | 32.5 at -33 °C | |
| Basicity | 4.75 | |
| Conjugate acid base pair | Acid | Base |
| ammonium | amide | |
| Molecular shape | Trigonal pyramid | |
| Dipole moment | 1.42D | |
Weakly boded ammonium hydrates have been isolated at low temperatures containing three dimension hydrogen bonding network of H2O molecules crossed linked with NH3 Molecules.
Concentrated aqueous solutions or liquor ammonia contain about 35 percent of NH3 by weight. Bottles of liquor ammonia developed high pressure, particularly in summer. Therefore, it should be cooled under the tap and covered with a towel before opening. Liquor ammonia is extremely damaging to the eyes. Common suppliers are supplied mostly 17N NH3 solutions.
Chemical Reactions
Ammonia burns in the air to produce nitrogen gas. In the presence of a platinum catalyst, it produces NO at 750 to 900 °C. It can be further oxidized to form NO2 for industrial production of nitric acid by the Ostwald process.
4NH3 + 5O2 → 4NO + 6H2O
A mixture of NH3 and CH4 forms HCN and H2 over a platinum catalyst at 1200 to 1500 °C.
CH4 + NH3 → HCN + 3H2
NH3 burns in fluorine to form NF3. Excess chlorine reacts with ammonia to form NCl3 while excess NH3 produces nitrogen and NH4Cl. The reaction of Cl2 with excess aqueous ammonia produces nitrogen gas. Chloramine is formed as an intermediate at pH scale 8. At pH 3 to 5, NHCl2 is formed.
| At pH > 8 | NH3 + OCl– → NH2Cl + OH– |
| At pH 3 to 5 | 2NH2Cl + H+ → NHCl2 + NH4+ |
| At pH < 3 | 3NH2Cl + H+ → NCl3 + NH4+ |
Red hot carbon reacts with ammonia to give NH4CN. Red hot metal oxides like PbO or CuO are readily reduced by NH3. Sodium metal reacts to form sodamide (NaNH2). Calcium, magnesium, and zinc also react with NH3 to form their nitrides.
Production Process
Ammonia is conventionally prepared by heating ammonium salt (NH4Cl) with a base like calcium hydroxide. Hydrolysis of metal nitrides provides a route for the production of 15NH3 or ND3.
3Mg + 15N2 → Mg315N2 + H2O → 215NH3 + 2Mg(OH)2
3Mg + N2 → Mg3N2 + D2O → 2ND3 + 2Mg(OD)2
Haber Process
Over 100 million tonnes of ammonia are now produced each year all over the world mainly by direct synthesis or Haber’s process. The process is favored at high pressure and low temperature. The chemical catalyst iron is used because it gives a higher yield and appreciable rate of conversion.
- The iron catalyst is prepared by fusing magnetite (F3O4) with KOH in the presence of small amounts of refractory catalysts like MgO, Al2O3, and SiO2.
- Nitrogen for the Heber process is obtained by fractionation of liquid air.
- Hydrogen is obtained by electrolysis of water or catalytic reforming of natural gas or methane.
Uses of Ammonia
Uses of Ammonia in Industry
A million tonnes of ammonia is manufactured and used each year over the world in wastewater treatment, leather, rubber, paper, food, and beverage industries. It can be oxidized on the surface of a hot platinum gauze catalyst to form NO. The NO can be converted to NO2 in the presence of oxygen and finally nitric acid. Therefore, the nitric acid produced from ammonia is used extensively in pharmaceuticals, dyes, and explosive industries.
In Agriculture
Ammonia is also used extensively in the synthesis of nitrogenous fertilizers like ammonium sulfate and phosphate in agriculture. About 90 percent of production is used in the agricultural field to grow the plants. For the preservation of fruits, it is also used as an antifungal agent.
Household Item
For the production of household cleaning products, we used wide amounts of ammonium solution. These products are used for cleaning mirrors, tubs, sinks, windows, etc. It is also used in fuel and antiseptic agents.
Production of Compounds
Ammonia is used for the production of various inorganic and organic compounds like nitric acid, hydrazine, hydroxylamine, urea, hydrogen cyanide, alcohol, amino acid, etc. Ammonia is also a useful solvent. Due to the low solubility of ionic compounds in liquid NH3, it may be used for the synthesis of various compounds like AgNO3 and KBH4.
Metal Analysis
Aqueous ammonium solution is used for quantitative analysis of metal ions. The effective concentration of OH– ion in solution may be controlled in the presence of ammonium salts like NH4Cl. Only the hydroxides of Fe (II), Cr (III), Al (III), and lanthanides are precipitated but Mg+2 and Zn+2 are not precipitated in the presence of ammonia.
Effects of Ammonia
Ammonia is highly toxic for our body, especially the eyes and skin. It reacts with water to form ammonium hydroxide which damages the body cells of our skin. Therefore, a concentrated aqueous solution or liquor ammonia effects our health. It effects mostly the eyes, nose, throat, and respiratory system of our body. It results in blindness, lung damage, or death. Bottles of liquor ammonia developed high pressure in summer and they can be removed after cooling.