What is Benzene?
Benzene is a class of organic compounds or aromatic hydrocarbons having the chemical formula C6H6. It is a flammable and colorless or slightly yellow liquid used for the production of several chemical compounds. Benzene is also used as a solvent in chemistry for many chemicals. Benzene has a regular hexagonal flat structure in which all the carbon atoms have equal charge density. The carbon atoms in the benzene molecule are located at the corner of the regular hexagon and six hydrogen atoms lie on the plane of the ring.
Benzene was first discovered by Faraday in 1926 from illuminating gas obtained from the pyrolysis of whale oil. Long-term exposure to benzene causes several types of harmful health effects such as cancer, leukemia, anemia, bone marrow defect, etc.
Structure of Benzene
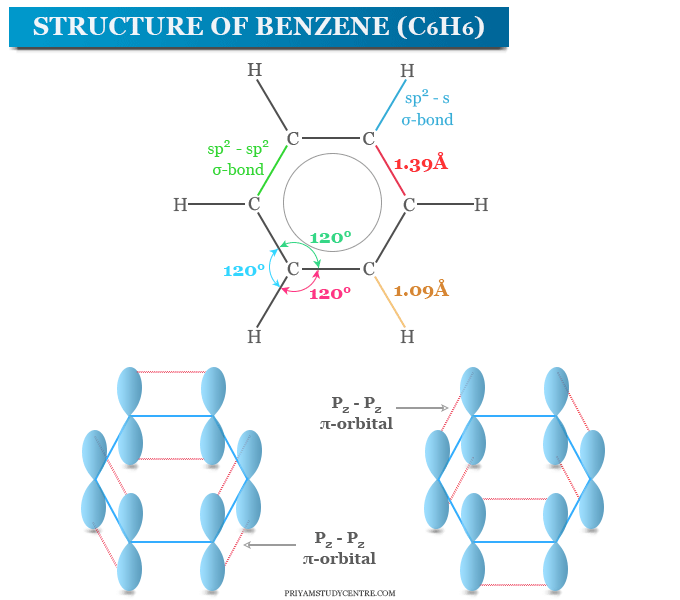
Analysis and molecular weight determinations show that the molecular formula of benzene is C6H6. All the carbon atoms in the benzene ring are sp2 hybridized. Each carbon atom has three sp2 atomic orbitals and one p orbital. Two sp2 orbitals of benzene form sp2-sp2 σ-bonds with two adjacent carbon atoms and one sp2 orbital forms carbon-hydrogen sp2-s σ-bond. Therefore, six carbon atoms form a planner ring in which C-H bonds are also co-planners.
The p-orbitals of six carbon atoms are parallel to each other and perpendicular to the plane. Any two of these p-orbitals can form a π-bond by side on overlapping. Therefore, all the bonds in benzene have a double bond character.
Properties of Benzene
Physical Properties
It is a colourless or light yellow organic compound with a peculiar smell. It is inflammable and burns with a smoky flame. Benzene is used as a good solvent for many chemical compounds. Therefore, it is used in dry cleaning.
| Properties | |
| Chemical formula | C6H6 |
| Molar mass | 78.114 g mol−1 |
| Appearance | Colorless or light yellow liquid |
| Odor | sweet aromatic |
| Density | 0.8765 g/cm3 |
| Melting point | 5.53 °C |
| Boiling point | 80.1 °C |
| Solubility | Soluble in water, alcohol, CHCl3, CCl4, diethyl ether, acetone, acetic acid |
| Molecular structure | Trigonal planar |
| Dipole moment | 0 D |
All the six hydrogen atoms in the benzene ring lie in the same plane and each C-C-H bond angle is 120°. This geometry of benzene has been established by x-ray analysis. It shows that all the C-C bond lengths (1.397 Å) are the same. This value lies between that of a single bond (1.54 Å) and a double bond (1.33 Å). Therefore, all the bonds in benzene have a double bond character.
Chemical Properties
It is a very stable compound which slowly attacked by a solution containing chromic acid or acid permanganate to form carbon dioxide and water.
It can be reduced catalytically to cyclohexane but partially hydrogenated to form dihydro and tetrahydrobenzene. Nickel and platinum are the best chemical catalysts for hydrogenation. Copper chromite or copper barium chromite is useful for the desired reduction of the side chains.
It is converted to a mixture of cyclohexane and methyl cyclopentane when heated with hydrogen iodide at 250 °C. The action of chlorine and bromine on benzene depends on the conditions applied for the reaction.
- In bright sunlight, chlorine adds on to form benzene hexachloride (C6H6Cl6).
- In the absence of direct sunlight, benzene undergoes a substitution reaction with chlorine. The reaction is slow but in the presence of a halogen carrier like iron or iodine, the substitution is rapid. Therefore, it forms chlorobenzene or dichlorobenzene when reacted with chlorine in absence of sunlight.
It forms nitrobenzene or benzene sulphonic acid when heated with concentrated sulfuric acid or nitric acid.
C6H5NO2 ← C6H6 → C6H5SO3H
Common Benzene Derivatives
All the six hydrogen atoms in benzene are equivalent. Therefore, only one mono-substituted derivative of benzene is possible. When two hydrogen atoms are replaced by two univalent groups, three disubstituted derivatives of benzene are possible.
- Positions 1, 2, and 1, 6 are equivalent. Therefore, 1, 2 or 1, 6-disubstituted derivatives are called ortho compounds.
- The 1, 3, and 1, 5 positions are equivalent. Therefore, 1, 3 or 1, 5-disubstituted derivatives are called meta compounds.
- The 1, 4-disubstituted derivatives are called para compounds.
Benzene Production
Benzene was first synthesized in 1870 by Berthelot by passing acetylene in a red hot copper tube. It may be prepared in the laboratory in many other processes. The most important laboratory production process is given below the diagram,
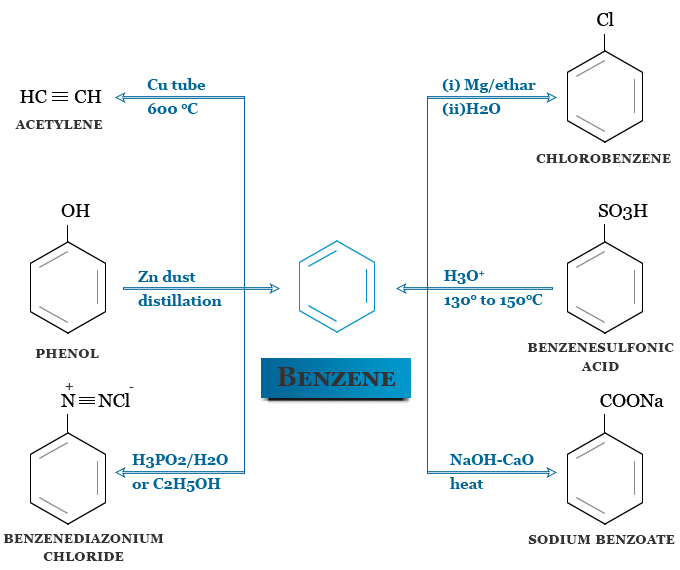
Aromatic compounds like Benzene, toluene, xylene, and ethylbenzene can also be extracted from petroleum where they occur naturally. They may be obtained by hydroforming and the thermal cracking of petroleum oils.
Hydroforming Process
Hydroforming is carried out under pressure at 480 to 550 °C in the presence of chromium oxide on an alumina support. Benzene, toluene, xylene, and ethylbenzene are obtained by hydroforming n-hexane, n-heptane, and n-octane.
Various aromatic hydrocarbon obtained by the hydroforming process is separated by a selective solvent process but benzene is obtained by this process in far less amount. Therefore, toluene, xylene, and ethylbenzene are converted to benzene by heating with hydrogen under pressure in the presence of a metal oxide catalyst. It is called hydrodealkylation.
C6H5CH3 + H2 → C6H6 + CH4
Thermal Cracking
Thermal cracking is an extraction or refining process in which high temperature and pressure are used to break down, rearrange, or combine hydrocarbon such as crude petroleum oil. Benzene, toluene, xylenes, naphthalene, anthracene, and many other polynuclear hydrocarbons have been isolated by the thermal cracking of alkanes.
Uses of Benzene
Benzene is a major component of gasoline. Therefore, it is found naturally in crude oil. In 1988, most of the chemicals listed on the American Chemical Society (ACS) contained at least one benzene ring. Therefore, it is used in motor fuel and makes several industrial chemicals like rubbers, lubricants, dyes, detergents, drugs, explosives, and pesticides.
The most common uses of benzene are given below,
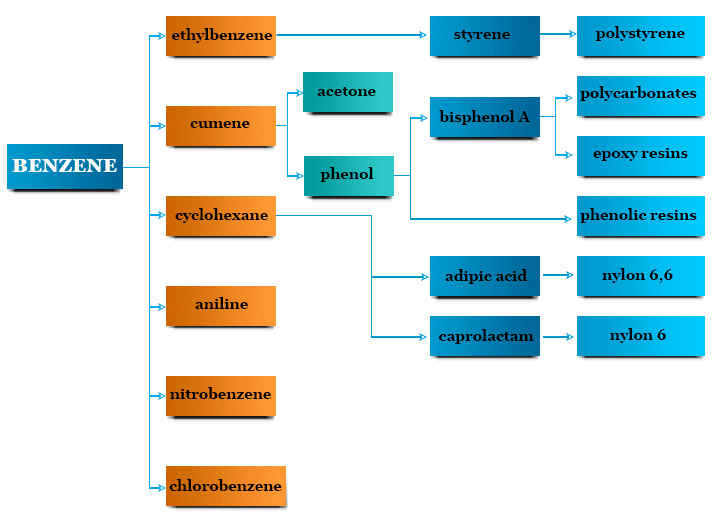
- Most of the benzene produced in the world is used for making ethylbenzene which is an important chemical used for making styrene, polystyrene, and different types of synthetic rubber.
- Cyclohexane obtained from benzene production is used for the manufacture of nylon fibers for textile engineering and the plastics industry.
- It is an important component of the production of nitrobenzene, aniline, and phenol. These compounds are used for the manufacture of pesticides like DDT and benzene hexachloride.
- Benzene uses as a liquid solvent for many chemicals like fats, oils, resin, rubber, sulfur, iodine, phosphorus, etc.
- Smaller amounts of benzene are used to make some industrial chemicals such as lubricants, dyes, detergents, drugs, explosives, etc.
Health Effects of Benzene
- Long-term exposure to benzene can affect mostly our blood. Therefore, it decreases red blood cells in our body to form anemia.
- A high level of benzene causes irregular menstrual periods and decreases ovaries in women.
- It also affects the development of fetuses in pregnant women or fertility in men.
- It has carcinogenic properties. Therefore, Long-term high levels of exposure to benzene cause cancer and leukemia in humans.