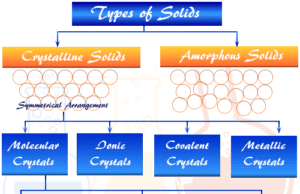
Material Used in Semiconductor
Semiconductor material is a substance which has electrical conductivity between conductors and a non-conductor or insulator. It is pure elements such as silicon, germanium, tin, and gallium or compounds such as gallium arsenide (GaAs), copper oxide (Cu2O), and iron oxide (FeO). Semiconductor material is used in most types of electronic devices such as laser diodes, solar cells, integrated circuits, etc. Unlike conductors, electrical conductance in semiconductors increases with increasing temperature. In the presence of impurities, an insulator shows semiconducting properties. Therefore, when very pure silicon or germanium is doped with a small amount of another periodic table element like boron, aluminum, phosphorus, arsenic, or antimony, it forms an n-type or p-type semiconductor.
Examples of Semiconductor
Non-stoichiometric compounds are excellent examples of semiconductors. A non-stoichiometric compound is one where the number of cations and anions is different. A crystal lattice has overall electroneutrality.
If there is a deficiency of cations, some of those cations present in the crystal lattice must have a higher positive charge. Ferrous oxide (FeO), ferrous sulfide (FeS), and copper oxide (Cu2O) are examples of cation-deficient non-stoichiometric compounds that act as a semiconductor.
![]()
If there is anion deficiency due to some excess metal ions, the lost electrons are trapped in the lattice. Zinc oxide (ZnO) and cadmium oxide (CdO) belong to non-stoichiometric anion deficient semiconductors.
Properties
The properties of semiconductors are between the conductors and insulators. It can be derived by following the band diagram,
![]()
- An insulator or a non-conductor has a completely filled energy band or valence band separated from the next energy band or conduction band by a finite energy gap. Under such a situation, electrons from the filled energy band cannot move onto the next energy band.
- In a semiconductor, the energy gap between the filled band and the vacant band or conduction band is very small. A very small amount of energy or electric field is required to excite electrons from the filled level to the lower levels of the vacant band. It is the fact of semiconductor to show conducting properties.
It is not necessarily true that only non-stoichiometric compounds show semiconducting properties. Very pure elements may be deliberately doped with a small number of other elements in order to create a deficiency in the number of electrons in the crystal lattice. When very pure germanium or silicon is doped with arsenic, it forms an n type semiconductor. If very pure germanium or silicon is doped with gallium, it forms a p type semiconductor.
Types of Semiconductor
The semiconductor can be classified into two types, intrinsic and extrinsic semiconductors.
Intrinsic Semiconductors
The conductor which conducts electricity in a very pure form is called an intrinsic semiconductor material. Pure germanium (Ge) or pure silicon (Si) are the most common type of intrinsic semiconductors. These are insulators at absolute zero temperature but exhibit conduction properties at rising temperatures.
When the temperature rises, some electrons move through the lattice due to collision. These free electrons and holes are responsible for the conduction of electricity on these types of semiconductors.
Extrinsic Semiconductors
An extrinsic semiconductor material is obtained by doping a specific impurity for the conduction of electricity through it. The process of adding impurities to a semiconductor is called doping. Usually, the conduction properties of intrinsic semiconductors are very poor and we cannot use them in electronic devices. Therefore, we add some impurities within intrinsic semiconductors to increase conduction properties.
According to the types of impurities or doping, extrinsic semiconductors are two types,
- n type semiconductor
- p type semiconductor
n Type Semiconductor
n type semiconductor is an extrinsic semiconductor formed when very pure silicon or germanium is dropped with a group-15 periodic table element such as phosphorus (P), arsenic (As), or antimony (Sb).
![]()
Silicon has four electrons while phosphorus has five electrons in their respective outer electronic shells. Therefore, every substitution of silicon by phosphorus gives an extra election to the silicon crystal lattice. Such additional electrons go to the unfilled energy band of silicon. In the application of an electric field, these electrons carry current and move within the crystal.
Anion deficient non-stoichiometric compounds have excess metal ions along with electrons trapped inside the lattice. These electrons are present in the lowest levels or vacant bands of non-stoichiometric compounds. On application of an electric field, the electrons move inside the vacant band carrying a current. Therefore, they show n type semiconductivity, n indicating negatively charged electrons. Non-stoichiometric ZnO and CdO are examples of n type semiconductors.
p Type Semiconductors
A p type semiconductor is an extrinsic semiconductor formed when very pure silicon or germanium is dropped with a group-13 periodic table element such as boron (B), aluminum (Al), or gallium (Ga).
![]()
Silicon has four electrons while boron has three electrons in their respective outer electronic shells. When pure silicon is doped with boron, there is a shortage of one electron for each boron atom substitution. They create a hole due to doping by boron. The hole which is created in the crystal lattice carries a positive charge. These holes are moving around the lattice to show semiconductivity. It is a p type semiconductor because the carrier hole is positively charged.
Non-stoichiometric cation deficient compounds such as FeO, CoO, and Cu2O have some cations with higher positive charges. These are examples of compounds that show p type semiconductivity.
Uses of Semiconductors
Semiconductors devices like diodes, microchips, and transistors are used widely due to their small size, solid-state devices, are easily portable, and have less input power for operation. They are used in our daily lives for the following purposes,
- Semiconductors are the common component that we used in many electronic devices such as calculators, mobile phones, laptops, solar technology, 3D printing machines, temperature sensors, automatic cars, etc. Today, we use doped silicon or germanium in most types of semiconductors.
- n and p type semiconductors are used for making diodes, microchips, and transistors such as bipolar junction transistors (BJT), and field-effect transistors (FET).
- It plays an important role in communication systems of our daily life like bank ATMs, trains, and internet communication.
- The physical and chemical properties of a semiconductor material make them capable of designing LED displays, solar cells, space vehicles, robots, etc.