Silicon Element
Silicon is an important metalloid or chemical element in the carbon family or Group 14 of the periodic table with atomic number 14 and symbol Si. It is used as the principal building materials of our civilization in the form of stones, sand, and clay. Silicon is the second most abundant element after oxygen in the earth’s crust. The outer electronic configuration of the element is s2p2. Therefore, silicon has four electrons in the outer quantum shell. It forms a face-centered diamond-type cubic crystal lattice. Due to the larger size and weaker bond energy, the meting point of the element is lower than that of carbon.
Properties of Silicon
The trends of properties of group 14 elements may be largely understood from the valence shell electronic configuration.
Common Properties
Some important atomic and physical properties of silicon are summarized below in the table,
| Silicon | |||
| Symbol | Si | ||
| Discovery | Jöns Jacob Berzelius in 1824 | ||
| Name derived | The Latin word silex’ or silicis means flint | ||
| Allotropes | Amorphous Si, Crystalline Si | ||
| Common isotopes | 14Si28, 14Si30 | ||
| Oxidation number or states | 4, −4 | ||
| CAS number | 7440-21-3 | ||
| Periodic Properties | |||
| Atomic number | 14 | ||
| Atomic weight | 28.085 | ||
| Electron per cell | 2, 8, 4 | ||
| Electronic Configuration | [Ne] 3s2 3p2 | ||
| Block | p-block | ||
| Group | 14 | ||
| Period | 3 | ||
| Physical Properties | |||
| State at 20 °C | Solid | ||
| Melting point | 1414 °C, 2577 °F | ||
| Boiling point | 3265 °C, 5909 °F | ||
| Molar heat capacity | 19.789 J mol−1 K−1 | ||
| Crystal structure | face-centered diamond-cubic | ||
| Density | 2.3290 g/cm3 | ||
| Atomic Properties | |||
| Atomic radius (non-bonded) | 2.10 Å | ||
| Covalent radius | 1.14 Å | ||
| Electronegativity | 1.90 (Pauling scale) | ||
| Electron affinity | 134.07 kJ mol−1 | ||
| Ionization energy (kJ/mol) | 1st | 2nd | 3rd |
| 786.51 | 1577.13 | 3231.58 | |
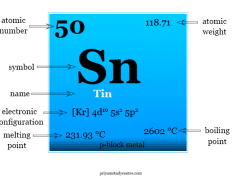
Silicon in Periodic Table
The chemistry and the periodic table position of silicon are followed by its electronic configuration.
![]()
The outer electronic configuration of the element is s2p2. It has four electrons in the outer quantum shell. Therefore, silicon is a typical metalloid of group 14 (IVA) of the periodic table.
Where is Silicon Found?
Silicon is the second most abundant element after oxygen found 27.6 percent in the earth’s crust but it cannot be found free in nature. It has a high affinity for oxygen forming the stable SiO4 unit which combines with another to form a variety of silicates.
Silica and silicates occur widely in the sand, clays, and various silicate minerals.
Production Process
It is produced by reducing SiO2 (sand) by high pure coke in an electric arc furnace
SiO2 + 2 C → Si + 2 CO
The formation of SiC was prevented by using excess SiO2. The product is nearly 96 to 97 percent pure. It was purified by converting SiCl4. The compound SiCl4 was purified by distillation and reduced by magnesium and zinc to produce pure silicon.
Super pure silicon is used in the electronics industry as a semiconductor and obtained by zone refining.
Interesting Facts
- It crystallizes like a diamond with a Si-Si bond distance in the crystalline solid equal to 235 pm. At very high pressure, a denser distorted form may be produced, but the Si-Si bond distance remains practically unchanged.
- The sum of the four ionization energies is very high. Therefore, attaining a noble gas configuration by losing four electrons is unfavorable.
- In most cases, it is attained by the formation of four covalent bonding with sp3 hybridization.
- Multiple chemical bonding is also formed by using its vacant 3d-orbital and filled p-orbital with oxygen, nitrogen, or halogen atoms.
Silicon Compounds
Silicon compounds are stereochemistry different from their carbon analogs. For example, (SiH3)3N is planar but (CH3)3N is tetrahedral.
The chemical behavior and compounds of Group-14 elements dominated under the + 4 oxidation state.
Hydride
All the elements of Group-14 formed covalent volatile hydrides but the tendency of formation decreases down the group.
Due to the strong tendency of catenation carbon formed a vast number of ring and chain compounds or hydrocarbons like alkanes or paraffin (CnH2n+2), alkenes or olefins (CnH2n), acetylene (CnH2n-2), and aromatic compounds.
Silicon formed a few saturated hydrides or silanes with the common chemical formula SinH2n+2.
Acid hydrolysis of magnesium silicide (Mg2Si) gives a mixture of SiH4, Si2H6, Si3H8, and Si4H10. The hydrides are separated and purified by fractional distillation.
What is Silicon Dioxide?
Silica or SiO2 is the most common dioxide of silicon. It is a yellow color oxide due to the presence of iron (II) oxides like granite and sandstone.
Many oxides are used in gemstones are basically hydrated SiO2. Rose quartz (pink), morion (dark brown), amethyst (violet), and citrine (yellow) are examples of such types of gemstones.
Silica also occurs in different types of vegetables and animal organisms like the straw of cereals, bamboo cane, and sponges.
Silica has different types of macromolecular structures built up by tetrahedral SiO4 units joined by the sharing of oxygen atoms. The two principal forms of silica are quartz and cristobalite.
Silicon Tetrahalides
Silicon tetrahalides may be prepared by the direct reaction of the element with a halogen molecule.
SiF4 is conveniently prepared by heating a mixture of CaF2 and silica in the presence of concentrated sulfuric acid. Unlike the halides of carbon, silicon tetrahalides are rapidly hydrolyzed by water and alkali metals.
The halides like SiF4 are partially hydrolyzed by water due to a secondary reaction between HF and SiF4 to produce hexafluorosilicate (SiF6−2).
Uses of Silicon
- Most of the silicon compounds are used industrially without being purified. More than 90 percent of the Earth’s crust contains silicate minerals, which are the compounds of silicon and oxygen.
- Many of these compounds like clays, silica sand, and most kinds of stone can be commercially used in building materials of our civilization.
- Calcium silicates are generally used in making Portland cement in building mortar and modern stucco.
- Ferrosilicon is used extensively for making corrosion-resistant steel.
Silicon Semiconductor
Small quantities of ultra-pure silicon are used in the electronic industry for making semiconductors. It formed an n-type semiconductor when doped with group-15 elements like phosphorus and arsenic given below the picture.
![]()
When silicon is doped with group 13 elements like boron or aluminum, it forms an n-type semiconductor.
![]()
These semiconductors controlled the conduction of electricity in electronic devices. Therefore, these types of semiconductors are used in almost all electronic devices like transistors, diodes, photosensors, microcontrollers, and integrated chips.