Tin Element
Tin is a chemical element or group 14 metal of the periodic table with the symbol Sn and atomic number 50. It is an important metal used in the early days of human civilization. Tin is used mainly for making non-toxic corrosion-resistant coating in steel, particularly for the packaging of food. The +2 oxidation number or state is common for tin and lead due to the inert pair effect. Tin has two crystalline solid allotropic forms and the common form is white metallic β-tin having a destroyed tetragonal close-packed crystal lattice with a density of 7.265 g/cm3. Another form is gray metallic α-tin which has a much lower density (5.769 g/cm3).
Where is Tin Found?
Tin is slightly more abundant than germanium but it contains specific ores like cassiterite or tinstone (SnO2). Small quantities of Sn are found in sulfides like stannite, cylindrite, franckeite, canfieldite, and teallite.
Tin scrap is also an important secondary source of metal. Recovery or recycling of scrap tin is increasing rapidly due to the huge global demand.
Malaysia, Russia, Bolivia, Indonesia, Peru Thailand, Brazil, and China are the major producer of the element Sn. In India, tinstone is found mostly in Bihar and Orissa.
Isotopes
Tin has ten stable isotopes with mass numbers 112, 114, 115, 116, 117, 118, 119, 120, 112, and 124. Of these 120Sn, 118Sn, and 116Sn are the most stable.
The isotope 119Sn (nuclear spin 1/2) is used for NMR studies and Mossbauer spectroscopy.
Production of Tin
The mineral cassiterite or tinstone contains only 1 to 5 percent of SnO2, together with silica and wolframite. The major steps for extraction of metal are,
- The ore is crushed and washed. Light siliceous matter is largely removed.
- The washed ore roasted in a current of hot air. Sulfur and arsenic compounds are driven out. Iron forms Fe3O4.
- The nonmagnetic tinstone is separated from magnetic wolframite and Fe2O3 by the magnetic separation technique. SnO2 is concentrated by further washed.
- The concentrate or black Sn is reduced with coke at 1200 to 1300 °C to produce Sn metal.
Properties of Tin
The chemistry and chemical properties of group 14 elements follow from their electronic configuration. All the elements contain an s2p2 valence shell electronic configuration. The elements Ge, Sn, and Pb have d and f electrons. Therefore, the properties of these elements differ widely from carbon and silicon.
The +2 state is the common and stable oxidation state of tin and lead but for other elements +4 state is common.
It liberates hydrogen from hot concentrated hydrochloric acid but concentrated sulfuric acid gives SnSO4 and sulfur dioxide.
| Tin | |||
| Symbol | Sn | ||
| Discovery | Approx 2100BC | ||
| Name derived from | From the Anglo-Saxon tin | ||
| Allotropes | White, gray, rhombic Sn | ||
| Main isotope | 120Sn | ||
| Periodic properties | |||
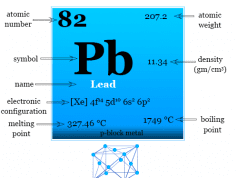
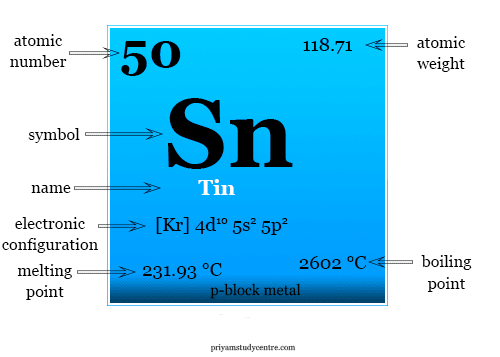
| Atomic number | 50 | ||
| Electron per shell | 2, 8, 18, 18, 4 | ||
| Electronic configuration | [Kr] 4d10 5s2 5p2 | ||
| Atomic weight | 118.71 | ||
| Group | 14 | ||
| Period | 5 | ||
| Block | p-block | ||
| Physical properties | |||
| Appearance | |||
| State at 20 °C | Solid | ||
| Melting point | 231.93 °C | ||
| Boiling point | 2602 °C | ||
| Density (g/cm3) | white, β-Sn | gray, α-Sn | |
| 7.265 | 5.769 | ||
| Molar heat capacity | 27.112 J mol−1 K−1 (white, β-Sn) | ||
| Electrical resistivity | 115 nΩ m | ||
| Crystal structure | White, β-Sn | Gray, α-Sn | |
| body-centered tetragonal | face-centered diamond-cubic | ||
| Chemical properties | |||
| Atomic radius (non-bonded) | 2.17 Å | ||
| Covalent radius | 1.40 Å | ||
| Common oxidation state | +2, +4 | ||
| Electron affinity | 107.298 kJ mol−1 | ||
| Electronegativity | 1.96 (Pauling scale) | ||
| Ionization energy (kJ/mol) |
1st | 2nd | 3rd |
| 708.6 | 1411.8 | 2943.0 | |
| CAS number | 7440-31-5 | ||
Tin in the Periodic Table
Tin is found in period 5 and group 14 or the carbon group of the periodic table.
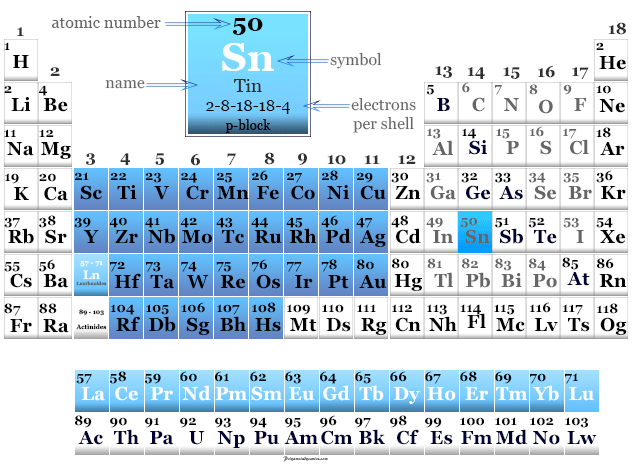
It is a p-block element that lies in between germanium and lead in the periodic table.
Chemical Compounds
Tin Hydride
All the elements of group 14 form gaseous hydrides like MH4. The stability of the group 14 hydrides decreases sharply from Ge to Pb.
SnH4 decomposes slowly at room temperature and is passive towards dilute aqueous acids and alkalis. But it is also decomposed by concentrated acids or alkalis.
SnH4 is a good reducing agent in organic chemistry. It is used for the transformation of benzaldehyde to benzyl alcohol or nitrobenzene to aniline.
Halides
All four tetrahalides of tin are known. Except for SnF4, all these halides are formed by covalent bonding with volatile in nature.
SnF4 is a hygroscopic white solid formed by treating SnCl4 with anhydrous HF.
The other SnX4 compounds are best made by the direct union of metal and halide. SnCl4 and SnBr4 are colorless liquids while SnI4 is a bright orange solid.
As mentioned earlier, the divalent state of Sn is more stable than the tetravalent state. Therefore, Sn forms all the stable dihalides. The dihalide, SnF2 has been used in toothpaste but presently it is replaced by NaF due to the toxic nature of Sn.
Tin Oxide
Tin occurs in nature largely as cassiterite or tinstone (SnO2). It is a white color solid and amphoteric in nature.
The amphoteric nature of Sn is shown by its reaction with fused sodium hydroxide and concentrated sulfuric acid.
SnO2 + 2 NaOH + H2O → Na2Sn(OH)6
SnO2 + 2 H2SO4 → Sn(SO4)2 + 2H2O
Sn also formed stable divalent oxide with the molecular formula SnO. It is more basic than SnO2.
SnO is obtained by the thermal decomposition of stannous oxalate in the absence of air.
SnC2O4 → SnO + CO + CO2
Uses of Tin
- Tin is one of the important metals used in early civilizations. It is mainly used as a non-toxic corrosion-resistant coating on food packaging.
- Tin alloying with lead produces solder which is used mainly for joining pipes and electric circuits.
- Tin is used for coating lead, zinc, and steel to prevent corrosion.
- Tin-plated steel containers are widely used for food preservation.
- Sheet steel coated with a Pb-Sn alloy is used for the roofing of gasoline tanks.
- It combines with other elements to form different types of alloys. For example, pewter (Sn 90 – 95%), Sb (1 -8%), Cu (0.5 – 3%). It is used for making trays, plates, and trophies.
- An alloy with 90 to 95% Sn and Pb is used in organ pipes.
- Tin is sometimes used for making American and Canadian pennies. Copper is the major content in such coins.
- Niobium tin compounds are used for making coils. These types of coils are used for making superconducting magnets due to their high critical temperature.