Germanium Element
Germanium is a chemical element or hard, lustrous, grayish white metalloid of group-14 of the periodic table with the symbol Ge and atomic number 32. It is used mainly for making optical parts in the infrared spectrometer. The general trends in chemistry of group-14 or the carbon group follow the typical pattern of the boron group. The trend of periodic table properties of group 14 elements is largely understood by their electronic configuration. The effect of shielding electrons of d and f orbitals is predominant for Ge and Pb. The high melting point in carbon indicates high C-C bond energy with the diamond-type crystal lattice. Germanium also has a diamond-type crystal lattice but the larger size and weaker bond energy result in a lower melting point in Ge.
Where is Germanium Found?
It is not found widely in ores. Germanium is found with zinc and silver minerals. It is mined in Alaska, Tennessee, China, the United Kingdom, Ukraine, Russia, and Belgium.
Isotopes of Element
Germanium has five stable isotopes with mass numbers 70, 72, 73, 74, 76. Among these, 76Ge is radioactive in nature with a half-life of 1.78 × 1021 years.
The relative abundance of these isotopes is given below in the table,
| Isotopes of germanium | |
| Isotope | Abundance |
| 70Ge | 20.5% |
| 72Ge | 27.4% |
| 73Ge | 7.8% |
| 74Ge | 36.5% |
| 76Ge | 7.8% |
Properties of Germanium
It has a diamond-type crystal lattice. However, the larger size and weak bond energy result in a lower melting point than silicon or carbon. The electronegativity of Ge is slightly greater than that of silicon or tin.
The chemical reactivity and electrical conductance of group-14 elements increase in the group.
- The energy gap between bonding and antibonding orbitals in carbon is very large. Therefore, it is an insulator.
- The energy gaps in Si, Ge, and Sn are 108, 64, and 7.7 kJ/mol respectively. Therefore, they are good semiconductors.
Elemental germanium burns slowly in the air to form GeO2. It is insoluble in dilute acids or alkalies but dissolves in hot concentrated sulfuric acid or nitric acid.
| Germanium |
|||
| Symbol | Ge | ||
| Discovery | Discovered by Clemens Winkler in 1886 | ||
| Name derived from | The Latin name of Germany, Germania | ||
| Common isotopes | 32Ge73 and 32Ge74 | ||
| Periodic properties | |||
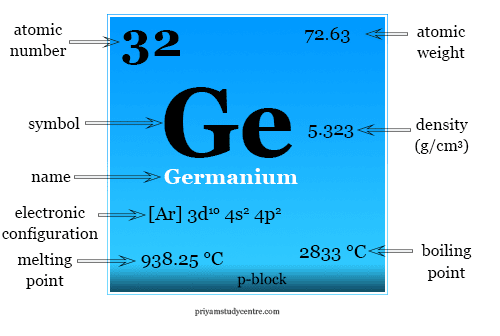
| Atomic number | 32 | ||
| Atomic weight | 72.63 | ||
| Electron per shell | 2, 8, 18, 4 | ||
| Electronic configuration | [Ar] 3d10 4s2 4p2 | ||
| Group | 14 | ||
| Period | 4 | ||
| Block | p-block | ||
| Physical properties | |||
| State at 20 °C | Solid | ||
| Melting point | 938.25 °C | ||
| Boiling point | 2833 °C | ||
| Density | 5.323 g/cm3 | ||
| Molar heat capacity | 23.222 J mol−1 K−1 | ||
| Electrical resistivity | 1.00 nΩ m | ||
| Crystal structure | face-centered diamond-cubic | ||
| Chemical properties | |||
| Atomic radius (non-bonded) | 2.11 Å | ||
| Covalent radius | 1.20 Å | ||
| Common oxidation number | +2, +4 | ||
| Electronegativity | 2.01 (Pauling scale) | ||
| Electron affinity | 118.94 kJ mol−1 | ||
| Ionization energy (kJ/mol) |
1st | 2nd | 3rd |
| 762 | 1537.5 | 3302.1 | |
| CAS number | 7440-56-4 | ||
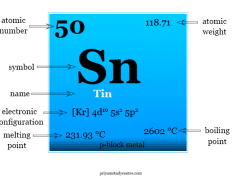
Germanium in Periodic Table
Germanium is found in period 4 and group 14 or the carbon family of the periodic table.
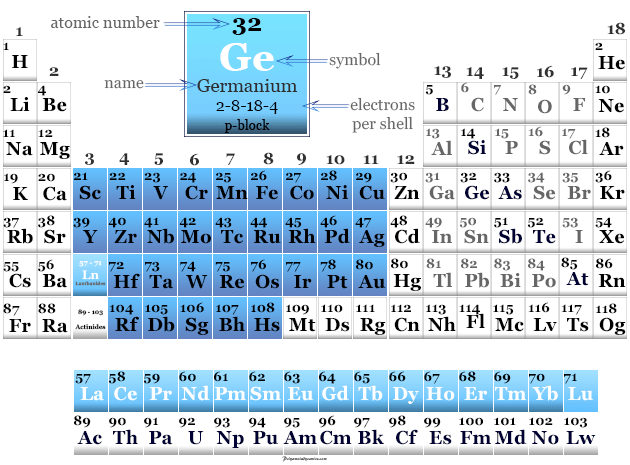
It is a p-block element that lies between silicon and tin in the periodic table.
Production Process
Previously, Ge was recovered from coal ash. Now it is mostly obtained from the flue dust of zinc.
- The flue dust is leached with dilute sulfuric acid and the solution is slowly neutralized by the gradual addition of sodium hydroxide. At pH scale 2.4, GeO2, xH2O is precipitated which is 98 percent complete at pH 5.
- The mixed precipitate is heated with hydrochloric acid when volatile GeCl4 is distilled out and ZnCl2 is left behind. The GeCl4 is purified by fractional distillation.
- GeCl4 hydrolyzed to form GeO2, xH2O.
- GeO2 is also reduced to form Ge by hydrogen at 550 °C.
- Pure germanium is produced by zone refining.
Germanium Compounds
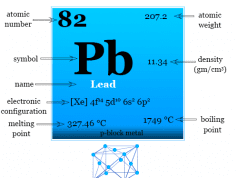
Most of the compounds of group-14 elements are formed in +2 and +4 oxidation states. The high values of ionization energy suggest that the formation of M+4 ions would be rather unlikely except for Sn and Pb.
The compounds of germanium are mostly formed by covalent bonding. Some important chemical compounds of Ge are given below,
Tetrafluoride
The tetrahalides of germanium are readily prepared from the elements or by the action of HX on GeO2. The physical properties of the tetrahalides are given below,
| Compound | Nature | melting point (°C) | boiling point (°C) |
| GeF4 | colourless gas | − 36.5 | − 15 |
| GeCl4 | fuming liquid | − 49.5 | 86.5 |
| GeI4 | colourless liquid | 26.1 | 186.5 |
| GeBr4 | red crystal | 144 | 440 |
The tetrafluoride is prepared by the reaction of concentrated HF with GeO2. It is a colorless hygroscopic gas with a boiling point of −36.5 °C.
Tetrachloride
Germanium tetrachloride is made by heating Ge in a stream of chlorine or by heating GeO2 with fuming hydrochloric acid. It is a fuming liquid with a melting point of − 49.5 °C and a boiling point of 86.5 °C.
Germanium Oxide
Germanium oxide is a white solid with the chemical formula GeO2. It is sparingly soluble in water. GeO2 is weakly acidic in nature, forming germinates with alkalies. It is forming GeCl4 with concentrated HCl.
GeO2 may be prepared by strongly heating the metalloid in oxygen or reacting of metalloid with concentrated nitric acid.
Uses of Germanium
Modern developments in semiconductor technology began in the 1950s with the introduction of germanium transistors. The production of pure germanium is easier than that of pure silicon.
Methods to prepare ultrapure silicon were developed in the 1960s. Therefore, silicon transistors are now widely used due to their superior quality. Hence the use of germanium transistors is now declined.
- Presently, germanium is used for making optical parts of infrared spectrometers.
- The compound, Germania (GeO2) is used for making wide-angle camera lenses, microscopy, and the core part of optical fibers due to its high refraction index and low optical dispersion.
- Germanium dioxide is also used as a chemical catalyst for the polymerization or production of polyethylene terephthalate.
- Silicon germanium alloys or semiconductors are used for making high-speed integrated circuits.