Arsenic Chemical Element
Arsenic is the chemical element of group 15 or nitrogen group of the periodic table with the symbol As and atomic number 33. Among the group-15 elements, nitrogen is an essential constituent of amino acids, and phosphorus is essential for bone but arsenic is very toxic or poisonous. Pollution caused by arsenic is a serious problem for human health that causes cancer and skin lesions in a number of countries of the world. The usual level of arsenic in drinking water is 10 ppb but higher than the normal range found in the different regions of the world. It is used largely in various light-emitting diodes and laser windows.
Arsenic Poisoning
Arsenic poisoning occurs due to a high level of As in our bodies. The poisonous nature of As in soil and water has been known from a very early time.
The three major biochemical actions of arsenic are coagulation of proteins, complexation with enzymes, and interference with phosphorylation (an important step for the generation of ATP).
- Short-term arsenic poisoning causes problems like vomiting, abdominal pain, encephalopathy, and diarrhea.
- Long-term poisoning is the most dangerous for our health. It can cause cancer, heart disease, diabetes, and darker skin.
The most common reason for long-term exposure is drinking water that contains a high level of As.
Prevention
- Marking the area as unsafe where the arsenic level is high in groundwater.
- Using a digital arsenic test kit to identify the safe zone for drinking water.
- By using water filters in homes to reduce the arsenic levels in drinking water.
- Take care when harvesting rainwater.
- Reducing the use of pesticides that contain high levels of As.
Arsenic Groundwater Contamination
The source of As in the groundwater is definitely natural for some parts of the world. The contamination also increases due to the mining of metal or the use of agricultural pesticides.
Arsenic in groundwater is much more in the long land of West Bengal and Bangladesh. More than 800,000 people from these areas drink arsenic-contaminated water.
Arsenic pollution in groundwater is not unique to West Bengal and Bangladesh. Many cases have been reported from Taiwan, Chile, North Mexico, and Argentina. Other minor incidents of arsenic contamination in groundwater have been reported in the USA, Canada, New Zealand, Japan, etc.
Where is Arsenic Found?
It is found very low less than 2 ppm in the earth’s crust. It occurs mainly as sulfides like realgar (As4S2), orpiment (As2S3), and metal arsenides like niccolite (NiAs), cobaltite (CoAsS), etc.
Arsenolite (As2O3) is also widely distributed throughout the world. Orpiment and realgar are found widely in the morain of the Shunkalpa glacier in the Kumaon Mountains.
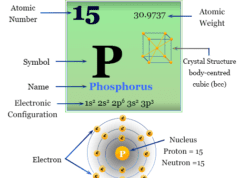
Properties of Arsenic
As, Sb and Bi contain tetramers. Rapid condensation of vapour formed an unstable yellow M4 unit. All three elements are brittle solids with colours varying from steel grey (As) to bluish-white (Sb) to dull white (Bi).
| Arsenic |
|||
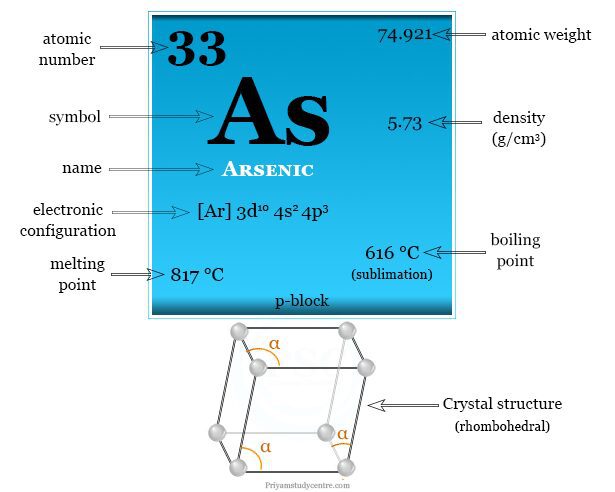
| Symbol | As | ||
| Discovery | Discovered by Albertus Magnus approx 1250 | ||
| Name derived from | Arsenikon, the Greek name for the yellow pigment orpiment | ||
| Allotropes | Yellow, Grey, and Black As | ||
| Key isotope | 75As | ||
| Periodic properties | |||
| Atomic number | 33 | ||
| Atomic weight | 74.921 | ||
| Appearance | metallic grey | ||
| Electron per shell | 2, 8, 18, 5 | ||
| Electronic configuration | [Ar] 3d10 4s2 4p3 | ||
| Group | 15 | ||
| Period | 4 | ||
| Block | p-block | ||
| Physical properties | |||
| Appearance | |||
| Density (g/cm3) | For solid | For liquid | |
| 5.73 | 5.22 | ||
| State at 20 °C | Solid | ||
| Melting point | 817 °C | ||
| Boiling point | 616 °C (sublimation) | ||
| Critical temperature | 1673 K | ||
| Crystal structure | rhombohedral | ||
| Chemical properties | |||
| Atomic radius (non-bonded) | 1.85 Å | ||
| Covalent radius | 1.20 Å | ||
| Common oxidation number | −3, +3, +5 | ||
| Molar heat capacity | 24.64 J mol−1 K−1 | ||
| Electronegativity | 2.18 according to the Pauling scale | ||
| Electron affinity | 77.574 kJ mol−1 | ||
| Ionization energy (kJ/mol) | 1st | 2nd | 3rd |
| 947.0 | 1798 | 2735 | |
| CAS Number | 7440-38-2 | ||
It has a similar electronegativity and ionization energies to that of phosphorus. It forms covalent bonding with most of the nonmetals. The elements also form a wide variety of binary compounds with every metallic element.
As in the Periodic Table
Arsenic is found in period 4 and group 15 or the nitrogen group of the periodic table.
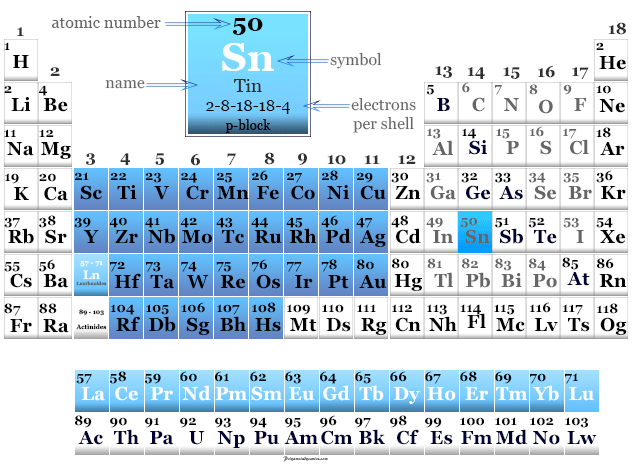
It is a p-block element that lies in between phosphorus and antimony in the periodic table.
Production Process
It is obtained by roasting arsenopyrite minerals in the absence of air and condensing the sublimate. Some As is trapped in the sulfide residue.
4 FeAsS → 4 FeS + As4(g)
It is converted to volatile As2O3 by roasting in the air. Flue dust of lead and copper obtained from their sulfide minerals is another source of As2O3. It may be reduced to the element by carbon reduction.
2 As2O3 + 6 C → 4 As + 6 CO
What are the Uses of Arsenic?
- Arsenic compounds are widely used in the early 20th century for controlling weeds and pests due to their toxicity. In 1980 all arsenate pesticides were banned due to the carcinogenic properties of As.
- It is still used for the preservation of wood and for making special types of glasses.
- In medicine, the arsenic-based drug Salvarsan was used for the treatment of syphilis until penicillin was discovered.
- Today arsenic trioxide is very effective for the treatment of promyelocytic leukemia.
- Elemental arsenic is used to make various alloys with lead. Its presence increases the fluidity, luster, strength, and hardness of the alloys.
- Today, GaAs and InAs are largely used as light-emitting diodes (LED) and laser windows.