Gas Chromatography Instrumentation
Gas chromatography (GC) in analytical chemistry is a common type of chromatography analysis process where the mobile phase is a gas and the solute of the analyzed sample is separated as a vapour. The mobile phase which we usually use in gas chromatography is noble gas or an unreactive gas such as helium, argon, nitrogen, and hydrogen. The main working principle of gas solid chromatography (GSC) or gas liquid chromatography (GLC) instrumentation is based on the movement of different components of the sample through a stationary phase present in the column. A gas chromatography system provides great advantages in testing the purity of substances or the separation of a particular component from a mixture of analyzed samples. The instrumentation of the gas chromatography machine is given below the picture,
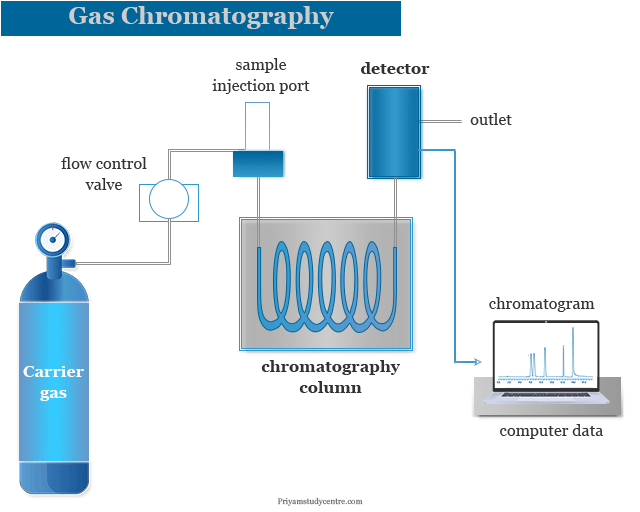
A sample is injected into a heating block where the analyzed molecule is vaporized. GC got a boost when gas liquid chromatography (GLC) was invented with good instrumentation. The success or failure of GC analysis or separation depends mainly on the retention time.
Gas Chromatography Machine
A good gas chromatography machine contains the following important components,
- Pressure regulator
- Sample injection port
- GC column
- Stationary phase
- Mobile phase
- Detector
- Signal recorder
Pressure Regulator
Pressure is adjusted within the limits of 1 to 4 atmospheres while the flow control valve measures 1 to 1000 liters per minute of gas. Flow valves are adjustable by a needle valve mounted on the base.
A trap containing a molecular sieve is used in GC instrumentation to filter out contaminating impurities. The preferred carrier gas may be helium, argon, nitrogen, and hydrogen due to their high thermal conductivity and low reactivity.
Sample Injection Port
Samples are injected by a microsyringe through a self-sealing silicon rubber septum in a heated metal. Generally, the metal bock is heated by an electrical heater fitted in the GC instrument. We used different sizes of injection ports for the injection of an analyzed sample.
Gas Chromatography Column
The gas chromatography column can be made by tubing coiled into an open spiral. Copper or stainless steel is used because they are stable during high-temperature operation. The choice of the column depends on the sample which we analyzed. The column in the gas chromatography instrument contains an electrically controlled oven.
The velocity of the carrier gas flow rate depends on the inner diameter of the chromatographic column. The usual size of the column is 2 meters. The short column is also used for the analysis of samples in the GC instrument. However, the separation in the short column is not possible.
Mobile Phase
The mobile phase is a chemically inert gas that is used to carry analyte molecules through the stationary phase of a heated column. The mobile phase which we usually use in GC instrumentation is noble gas or an unreactive gas such as helium, argon, nitrogen, and hydrogen.
Detector
The detector can detect the arrival of separated components coming from the column to provide an electrical signal. Pressure and temperature detectors are the two major groups of detectors used in GC analysis.
The detector in gas chromatography instrumentation is situated near the column to avoid the condensation of liquids or to detect the sample before decomposition. In a packed column GC instrumentation, we used mostly a thermal conductivity detector (TCD) or a flame ionization detector (FID). Among these TCD is the most popular. A flame ionization detector (FID) is most useful where the effluent is suitably attenuated by a stream splitter.
TCD detector contains four heat sensing elements made by thermistors or resistance wires. The thermometers are electronic semiconductors of fused metal oxides whose electrical resistance varies with temperature. The above types of detectors are useful because they have no direct contact with the gas stream.
Stationary Phase in Gas Chromatography
Gas liquid chromatography can be available in almost an infinite variety of liquid partition materials. The liquid or stationary phase in gas chromatography can be divided into nonpolar, intermediate polarity, polar carbowaxes, and hydrogen bonding compounds like glycol.
The maximum temperature of the stationary phase can be determined by its volatility. The excess volatility of the stationary phase can shorten the life of the column.
Loading of the column by stationary phase can be expressed by percentage of weight. For example, 15% means, a 100 g column has 15 g of stationary phase.
| Stationary Phase in GC Instrument | |
| Type | Stationary phase |
| GLC | Squalene, silicon oil, nonpolar polyethylene, glycol, glass Teflon beads, etc. |
| GSC (usual) | Silica gel, alumina, charcoal, molecular sieve inorganic salts, mineral, porous polymers. |
| GSC (reverse-phase) | Silica alumina coated with organic or inorganic compounds or complexes. |
Gas Chromatogram
The choice of recorder determines the ultimate accuracy of the gas chromatogram. The full-scale pass response should be 1 second. Sometimes amplification of signals is essential to give a gas chromatogram.
In the gas chromatogram, we have a Gaussian error function curve that is symmetrical. If a substance has no affinity for the stationary phase, the partition coefficient, K = 0. Therefore, it will not be retained by the column.
Principle and Working of Gas Chromatography
The main principle of gas chromatography instrumentation is based on the movement of different components of the sample through a stationary phase present in the column. The component of a sample that has a greater affinity for the stationary phase spends more time in the column. Therefore, it elutes later and has a longer retention time than the component that has a lower affinity for the stationary phase.
Separation in gas chromatography is feasible by partitioning the sample between a mobile gas phase and a thin layer stationary phase of nonvolatile, high boiling liquid held on the solid support. The idea of fractioning gases by passage over solid or immobilized gases was first introduced in 1941 and became popular after 1955.
- A sample is injected into a heating block where the compound is readily vaporized. The sample vapour is carried by the carrier gas into the column inlet.
- The solute is absorbed in the head of the column by the stationary phase. However, it travels at its own rate through the column according to its partition coefficient value.
- Solutes are eluted according to their partition coefficient and entered into the detector.
- In the detector, solutes give a series of signals resulting from concentration changes and different rates of elution.
- A plot or chromatogram of time against the composition of the carrier gas stream appears in the recorder of the GC machine. The peaks of the plot give the quantitative data in the gas chromatogram.
Applications of Gas Chromatography
Gas chromatography instrumentation is usually used for the identification and analysis of organic molecules and polymers. In elemental analysis, the class of pyrolysis reactions where the organic products break down into carbon dioxide and water can be carried out by a gas chromatography instrument. GC technique can be used in a wide number of industries with different types of applications.
Food Analysis
- GC is an important technique in food technology because it can be used widely for the analysis and separation of food products.
- The GC technique is used widely for quantitative and qualitative analysis of food.
- The technique is used mainly for the analysis of additives, flavors, and aromas added to food products.
- A GC machine can be used to detect toxic elements or compounds such as pesticides, fumigants, and naturally occurring toxins present in food. Therefore, the GC technique ensures the safety of food products.
Quality Control
The industries which produce cars, chemicals, and pharmaceuticals can use gas chromatography instrumentation for quality control of their products. Many chemical and pharmaceutical industries produce pure and large quantities of products by the application of the GC technique.
Research
Many new research papers can be published with the help of gas chromatography instruments. However, this technique is used mainly for analyzing natural products and meteorites that fall to Earth from space.
Forensic Analysis
The gas chromatography instrument is applicable in many forensic investigations and detections. For example, the causes and time of death can be investigated by GC instrument.
Air Pollution
Air pollution is a major environmental problem in today’s life. It can negatively affect nature and the health of human beings. GC instrumentation is used for monitoring levels of air pollution caused by different types of air pollutants such as carbon dioxide, nitrogen oxide, sulfur dioxide, sulfur trioxide, etc.
Advantages of Gas Chromatography
There are several advantages of gas chromatography. The most common advantages are,
- We used longer columns to give high separation efficiency. Gas chromatography columns are available in a wide range of diameters and lengths. Therefore, it offers a wide choice of applications for a wide range of separations.
- GC instrument has a wide range of freedom to change operational parameters such as chromatographic column, temperature programming, carrier gas flow rate, etc.
- A very convenient change of operational parameters during the gas chromatographic process gives high resolution in the shortest possible time for the operation. However, it is not available in any other chromatographic methods such as thin layer chromatography, column chromatography, ion exchange chromatography, etc.
- GC technique provides a very good speed from the low viscosity of gases or vapor.
- GC is ideal for the analysis of volatile compounds. Therefore, gas chromatography instrumentation is used for the analysis or separation of a wide range of chemical compounds.
Frequently Asked Questions
What is gas chromatography?
Gas chromatography (GC) in analytical chemistry is a common type of chromatography principle where the mobile phase is a gas and the solute of the analyzed sample is separated as a vapour. The mobile phase which we usually use in GC analysis is noble gas or an unreactive gas such as helium, argon, nitrogen, and hydrogen. It analyzed vaporized samples without any decomposition.
What is gas chromatography principle?
The main principle of gas chromatography is based on the movement of different components of the sample through a stationary phase present in the column. The column is made of a narrow tubing coiled into an open spiral.
The component of a sample that has a greater affinity for the stationary phase spends more time in the column. Therefore, it elutes later and has a longer retention time (Rt) than the component that has a lower affinity for the stationary phase. When a component exits from the column, it can be detected and identified electronically.
How does gas chromatography work?
Gas chromatography work by injecting analyzed samples into a heating block where the compound is readily vaporized. The sample vapour formed in the heating block is carried by the carrier gas into the column inlet. The solute is absorbed in the head of the column by the stationary phase and travels at its own rate through the column according to its partition coefficient.
When a component exits from the column, it can be detected by the detector. Generally, a detector in the GC system provides a series of signals resulting from concentration changes and different rates of elution. A plot or chromatogram of time against the composition of the carrier gas stream appears in the recorder of the GC machine.
What is gas chromatography used for?
Gas chromatography is usually used for the identification and analysis of organic molecules and biopolymers. It has uses mainly for food analysis, quality control, research, forensic and environmental analysis, etc.
What is the stationary phase in gas chromatography?
The stationary phase in the GC technique is either a solid adsorbent or a liquid with an inert support in which the mobile phase is moved. Today a polymeric liquid stationary phase is used in GC instruments. The stationary phase is placed inside a separation column of a gas chromatography instrument.