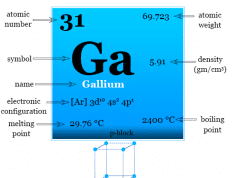
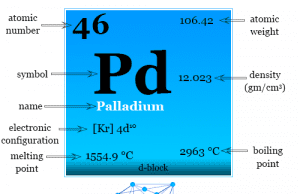
Different Types of Steel
Steel is essentially a refined alloy of iron that contains a lower percentage of carbon, silicon, and other metals to improve the mechanical strength of the metal. The mechanical and corrosion properties of steel and stainless steel are generally modified by changing composition and adding different types of transition metals like nickel, manganese, chromium, titanium, vanadium, tungsten, etc. It is used in different types of manufacturing and construction industries of the world. Steelmaking is mostly carried out by refining cast iron followed by the addition of alloying chemical elements like carbon, silicon, manganese, cobalt, nickel, chromium, etc. It is the most widely used building material in the world’s infrastructure and industries. Therefore, steel has been uses for the production of everything from sewing needles to large useable tools.
Composition of Steel and Stainless Steel
Stainless steel is the principal material or alloy of iron that is mostly used in different types of structural units. Mild steel is used generally for making most of the machine parts, tools, and utensils.
| Types of Steel | Composition | Uses |
| Silicon Steel | 3.5 percent silicon and very little carbon | Used to make electromagnets and transformers. |
| Stainless Steel | 10 to 12 percent Cr | Increase resistance, hardness, and corrosion properties, used for making high temperature and high-pressure equipment. |
| Manganese Steel | 12 to 13 percent of Manganese | Extremely hard and resistant to wear, used in rails, conveyors, chains, rocks crushers, or grinding machinery and safe. |
| Nickel Steel | 2 to 4 percent of nickel | High ductility and tensile strength, used for making cables, propeller shafts, gears, etc. |
| Cobalt Steel | up to 35 percent of cobalt | Highly magnetic, used for making permanent magnets. |
| Chrome-nickel Steel | 1 to 4 percent nickel and 0.5 to 2 percent chromium | High tensile strength and greater corrosion resistance, used for making motor car and bicycle parts. |
| Tungsten Steel | 10 to 20 percent tungsten and 3 to 8 percent chromium | Very hard, corrosion resistant, and retain temper at high temperature, used in making high-speed cutting tools. |
| Molybdenum steel | 6 to 7 percent molybdenum | Retain temper at high temperature and used for making high-speed cutting tools. |
Mechanical Properties
The iron-carbon and iron silicon phase equilibrium plays a vital role in the mechanical and technical strength of alloys of iron or steel. The varying properties and uses of steels by carbon are mostly associated with the formation or distribution of hard carbide (Fe3C) compounds or cementite.
Production of Steel
The steel production process consists of some general steps. First, we remove the carbon, sulfur, and phosphorus from molten pig iron by oxidation usually by oxygen. In this process, carbon and sulfur oxidize to form carbon dioxide and sulfur dioxide and escape. The impurities like manganese, silicon, and phosphorus also form oxides which combine with lime or calcium oxide to form slag.
Next, separate the slag followed by the addition of requisite quantities of deoxidizer and other alloying elements. A reducing agent like petroleum coke together with ferrosilicon and ferromanganese is used as the deoxidizer. They generally reduce any left FeO. The technique of steelmaking has undergone vast changes in the last fifty years. At present, it is going through the basic oxygen, open-hearth, and electric arc processes.
Basic Oxygen Steelmaking Process
- In the basic oxygen process, we used 99 percent pure oxygen for the production of steel from molten pig iron.
- The pure oxygen is blown onto the surface of molten pig iron at great speed through water-cooling pipes. The charge of hot molten pig iron and lime is taken in a converter lined with dolomite and magnesium oxide.
- The oxygen penetrates into the molten charge creating a large turbulence motion and oxidizing the impurities.
- The slag is separated by tilting the converter. A rapid analytical check is performed to bring the metal to its desired composition. The necessary addition of alloying metals to steelmaking.
Open Hearth Process
In this process, the steelmaking is carried out on the hearth of a furnace. It is heated mostly by burning gas or oil in air or oxygen. The hearth is generally lined with dolomite, magnesite, cast iron, and scrap iron with 5 to 6 percent lime and melted.
Oxygen is also injected into the furnace through a water-cooled pipe to remove the impurities. The process requires a much longer time than the basic oxygen process.
Electric Arc Furnace
Different types of high-grade steels are prepared in the electric arc furnace. It is going through the direct arc and indirect arc process.
- Direct arc furnaces are generally fitted with three electrodes. It penetrates the furnace crucible and strikes short circuits between their extremities and the molten charge.
- In indirect arc furnaces, the arc is struck between two large electrodes. The specific heat from the electrode is heated to the charge.
In different types of steel production processes, the charge consists of solid scrap iron, pig iron, coke, and anthracites if enough cast iron is not present. Melting and alloying of steel are also conveniently carried out in induction furnaces which contain a vertical basin around which an induction coil wound.