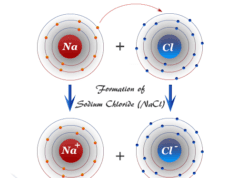
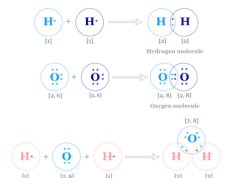
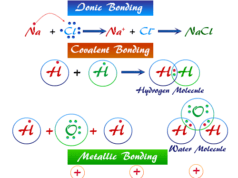
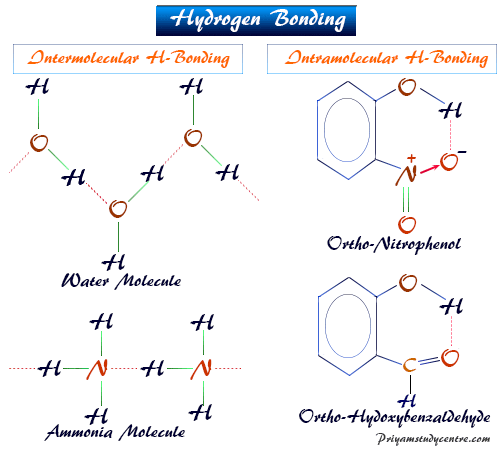
Hydrogen Bonding Examples
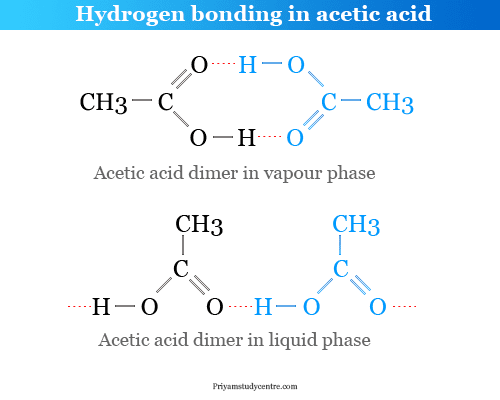
Hydrogen bonding or intermolecular and intramolecular hydrogen bond is a weak type of chemical bond due to very unstable attractive forces responsible for the formation of H-bond in chemistry. Common examples of intramolecular hydrogen bonding are seen in the dimer of formic acid, acetic acid, o-hydroxybenzaldehyde, o-nitrophenol, etc. Intermolecular hydrogen bonding is formed in water, ammonia, hydrogen fluoride, etc. The positive hydrogen end of the dipole can weakly link or bond with another negative dipole end present in the same molecule like o-nitrophenol or another molecule like water, ice, ammonia, etc. Some examples of the formation of hydrogen bonds are given above the picture.
Formation of H-Bonding
The electronic configuration of hydrogen is 1s1. The nucleus of hydrogen is surrounded by only one electron in 1s orbital with the maximum capacity of two electrons. Therefore, hydrogen has the capability of forming a single covalent chemical bond with another atom. If the covalently bonded atom is strongly electronegative, the bonds become polar.
This weak secondary link between two electronegative atoms through hydrogen atoms is defined as intramolecular or intermolecular hydrogen bonds.
Types of Hydrogen Bonding
It is mainly two types,
- Intramolecular hydrogen bonds
- intermolecular hydrogen bonds
Intermolecular Hydrogen Bonding
The hydrogen linking occurring between two similar or different molecules is called intermolecular hydrogen bonding. Water, ammonia, and hydrogen fluoride are examples of such types of molecules.
Hydrogen Bonding in HF
In hydrogen fluoride (HF), the positive end of one dipole attracts the negative end of another similar dipole. These molecules are associated together to form the cluster, (HF)n.
Hydrogen Bonding in Water
Hydrogen bonding in water leads to the molecular association of H2O molecules. Such types of molecular association of water form the polymerized molecule like (H2O)n in which hydrogen acts as a bridge between two highly electronegative oxygen atoms.
The hydrogen bond in water is important to the existence of our world. In the absence of hydrogen bonds, water would be a gas at ordinary temperature. Therefore, without liquid water, we cannot imagine animal or vegetable life.
In hydrogen sulfide, due to the small electronegativity of sulfur atoms, H2S does not form (H2S)n type cluster by H-bonding. Therefore, water exists in liquid form but hydrogen sulfide exists as a gas molecule.
Intramolecular Hydrogen Bonding
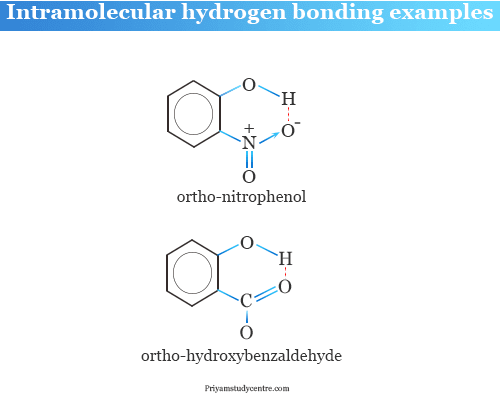
The hydrogen linking occurring within the single molecules is called intramolecular hydrogen bonding. Intramolecular H-bond gives rise to ring formation or chelation. Aromatic alcohol like o-nitrophenol defines an intramolecular hydrogen bond.
Application of Hydrogen Bonds
The unstable link or hydrogen bond uses to explain the unexpected physical properties like density, melting point, and boiling point of the solid, liquid, gaseous inorganic, and organic compounds.
For example, the crystalline solid form of phenol or carboxylic acid is supported due to the formation of the hydrogen bond.
o-Nitrophenol and p-Nitrophenol Boiling Point
o-nitrophenol boils at 214 °C, while m- and p-isomer boils at 290 °C and 270 °C respectively.
The hydrogen bond in o-nitrophenol is limited within the same molecule but p- and m-nitrophenol extends to the neighboring molecules. Hence the p- and meta nitrophenol gather a large number of the molecule through the hydrogen bond.
Ortho Hydroxybenzaldehyde
Ortho hydroxy benzaldehyde also restricted hydrogen-bond within the same molecule. It leads to the weakening acid properties of the compound.
Due to H-bonding, the scope for the formation of hydrogen ions in the solution is limited which increases the pH scale of the solution.
Properties of Hydrogen Bond
The bond energy of the H-bonding is in the range of 3 to 10 kcal mol−1 but in normal covalent bonds in the range of 50 to 100 kcal mol−1. Therefore, a normal covalent bond is a stronger bond than weaker hydrogen bonds present in inorganic or organic compounds.
It does not involve any sharing of the electron particles. Hence the characteristics of the hydrogen bond are quite different from the normal covalent bond.
Strength of Hydrogen Bond
- The strength of hydrogen bonds is directly related to the electronegativity and polarity of bonds between the periodic table elements.
- With the increasing electronegativity or electron affinity, the strength of hydrogen bonds also increases.
- For example, the H-bonding strength of ammonia < water < hydrogen fluoride.
- The hydrogen bond is detected by the electromagnetic spectrum in absorption spectroscopy, infrared spectrum, and x-ray methods.
Hydrogen Bonding in Ice
Ice is a crystalline solid. In the ice crystal lattice, the oxygen-atom tetrahedrally is surrounded by four hydrogen atom.
- Two hydrogen atoms are linked to the oxygen atom by a covalent bond.
- The remaining two hydrogen atoms are linked to the oxygen atom of other water molecules by hydrogen bonding.
In an ice crystal, every water molecule is associated with the other four water molecules by H-bonding in a tetrahedral fashion with a large amount of space.
Why density of water is maximum at 277K?
When ice heat melts from 0 to 4 °C or 277K, the hydrogen bonds are broken down and the space between water molecules decreases. The density of water is maximum at 4 °C or 277K.
Above 4°C, the kinetic energy of the molecules is sufficient to disperse from each other, and the concentration steadily decreases to form water due to breaking Hydrogen-bond.
Importance of Hydrogen Bonding
In chemical and biological science, hydrogen bonding is very important for the existence of our environment or our life. Due to the absence of hydrogen bonds, the liquid water or ice would be in the gas phase at ordinary temperatures.
Therefore, without liquid water or the absence of hydrogen bonding, we can not imagine the existence of animal or vegetable life in our environment.