Alcohol n Propanol
n-propanol also called n-propyl alcohol or 1-propanol or propan-1-ol is the primary alcohol with the chemical formula C3H8O. It is used widely in organic synthesis and solvents in the pharmaceutical and chemical industries. n-propanol is a colurless liquid that is completely miscible in water, ethanol, and ether. Oxidation of n-propyl alcohol with acidic dichromate gives propionaldehyde. Propanol has two isomeric forms, 1-propanol is one of the two isomers of propanol with the chemical formula written as CH3CH2CH2OH. The other isomer of propanol is isopropanol or isopropyl alcohol.
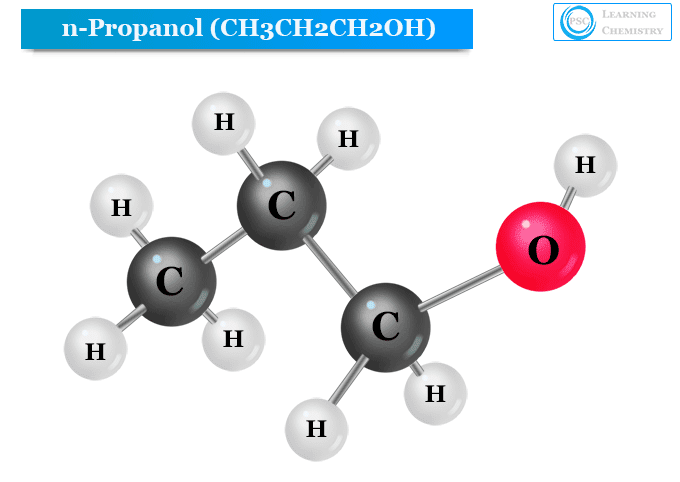
n-Propanol Structure
The chemical formula of propanol is written as C3H8O. Therefore, the structure of propanol (n-propanol and isopropanol) contains a three-carbon chain. The chemical formula of n-propanol is written as CH3CH2CH2OH. Therefore, the hydroxyl group of n-propyl alcohol is directly bonded to the primary carbon atom of the carbon chain.
Production Process
N-propyl alcohol or propan-1-ol or n-propanol was originally obtained from fusel oil. Now, a large amount of n-propyl alcohol can be produced by the catalytic hydrogenation of propionaldehyde.
Propionaldehyde can be produced by hydroformylation of ethylene by carbon monoxide and hydrogen in the presence of cobalt octacarbonyl.
H2C=CH2 + CO + H2 → CH3CH2CH=O
CH3CH2CH=O + H2 → CH3CH2CH2OH
A more recent production process of n-propyl alcohol is the catalytic reduction of propargyl alcohol. Propargyl alcohol is obtained by the addition of acetylene and formaldehyde in the presence of a copper catalyst.
CH≡CCH2OH + 2 H2 → 2 CH3CH2CH2OH
Naturally, 1-propanol is formed during many fermentation processes.
Properties
N-propyl alcohol is a colorless liquid with the smell of alcohol. It is miscible in water, ethanol, and ether. It is one of the two isomers of propanol.
Some common properties of n-propanol are given below the table,
| IUPAC name | Propan-1-ol |
| Other names | n-Propyl alcohol n-Propanol 1-Propanol n-PrOH Ethyl carbinol 1-Hydroxypropane Propionic alcohol Propionyl alcohol Propionylol Propyl alcohol Propylic alcohol Propylol |
| Chemical formula | C3H8O |
| Molar mass | 60.096 g/mol |
| Appearance | Colorless liquid with the smell of alcohol |
| Density | 0.803 g/mL |
| Melting point | −126 °C |
| Boiling point | 97.4 °C |
| Solubility | Completely miscible in water, ethanol, and ether |
| Dipole moment | 1.68 D |
It can participate in normal reactions of primary alcohol. Therefore, it can be reacted with an organic acid such as acetic acid in the presence of sulfuric acid to form propyl acetate.
CH3COOH + CH3CH2CH2OH → CH3COOCH2CH2CH3 + H2O
It can react with phosphorus halides to form propanol halides.
CH3CH2CH2OH + PCl5 → CH3CH2CH2Cl + HCl + POCl3
Alcohol may be oxidized. The products of oxidation depend on the types of alcohol and the nature of the oxidizing agent.
Propan-1-ol oxidized with Na2Cr2O7 and H2SO4 gives a 36% propionaldehyde. However, it can be oxidized to propanoic acid in the presence of chromic acid.
It may be dehydrated to alkenes by concentrated sulfuric acid at about 170 °C.
What is n-Propanol Used For?
- Most commonly n-propanol uses as a solvent for waxes, vegetable oils, inks, natural resins, synthetic resins, etc.
- 1-propanol can be involved in the synthesis of many organic compounds and intermediates to produce esters, halides, propyl amines, and propyl acetate.
- n-propyl alcohol may be used as a solvent for pharmaceutical products, polishing compositions, brake fluids, lacquers, printing inks, natural gums, pigments, dye solutions, antifreeze, and de-greasing fluids.
- We used n-propanol for the production of various daily-used chemical products such as cosmetics, cleaning products, perfumes, fragrances, printing, coatings, etc.
- It is also used as a fuel additive for engines due to its high octane number. But due to its expensive nature and low energy gaining, n-propanol or n-propyl alcohol is not uses in motor fuel.