Isopropyl Alcohol (2 propanol)
Isopropyl alcohol (2 propanol) also called isopropanol (chemical formula CH3CHOHCH3) is a colorless, flammable isomer of propyl alcohol that is used in various household and commercial products such as rubbing alcohol, cleaners, disinfectants, and hand sanitizers. It is also used to make several organic compounds such as esters, acetone, ketene, and high-octane fuel. It is made industrially by passing propene into concentrated sulfuric acid and diluting it with water. The toxicity of isopropyl alcohol is less than that of ethanol but considerably more than ethylene glycol or methanol. An isopropyl group is directly attached to a hydroxyl group in the structure of isopropyl alcohol or isopropanol molecule.
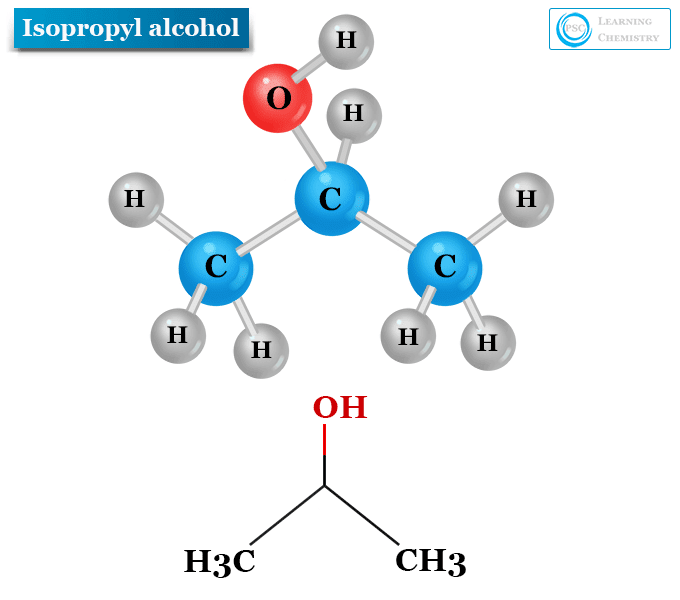
Structure of Isopropyl Alcohol
The chemical formula of isopropyl alcohol is C3H8O. Therefore, the structure of the isopropyl alcohol molecule is formed by bonding three carbon atoms, eight hydrogen atoms, and one oxygen atom.
- The three carbon atoms of C3H8O are bonded together to form a carbon chain.
- Each of the two terminal carbon atoms of the carbon chain is bonded with three hydrogen atoms.
- The middle carbon atom of the chain is bonded with one hydrogen atom and one hydroxyl group of isopropanol.
Other ways to write the chemical formula of isopropyl alcohol are C3H7OH, (CH3)2CHOH and CH3CHOHCH3. According to the above chemical formula, an isopropyl group is directly attached to a hydroxyl group in an isopropyl alcohol or isopropanol molecule.
How is Isopropyl Alcohol Made?
Propene is one of the basic materials used to make isopropyl alcohol. Naturally, propene has come from the refining of fossil fuels such as petroleum, natural gas, and coal. It is primarily made by indirect and direct hydration of propene with water.
- Indirect hydration of propene with concentrated sulfuric acid gives a mixture of sulfate esters. Subsequent hydrolysis of sulfate esters by steam produces isopropyl alcohol.
- It is also made by direct hydration of a mixture of propene and steam under pressure at 220 to 250 °C over a chemical catalyst of tungsten oxide plus zinc oxide on a silica carrier.
In both the hydration process, it can be separated from water or other by-products by distillation. Simple distillation gives 87.9 percent isopropanol (by mass) and 12.1 percent water (by mass). Very pure or anhydrous material is made by azeotropic distillation of the wet isopropyl alcohol.
Isopropanol can be prepared by the hydrogenation of acetone but it involves an extra step to make. Generally, acetone is prepared from propene through the cumene process.
The United States, Europe, and Japan are the most common producers of isopropanol in the world.
Properties of Isopropyl Alcohol
Propan-2-ol or isopropanol is a clear, colorless liquid with an alcoholic smell and a slightly bitter taste. It is soluble in water and many other organic solvents. It forms an azeotropic water mixture that contains 91% isopropyl alcohol.
Isopropanol is a toxic chemical compound that is not safe for drinking. Isopropanol gives the iodoform test and oxidation with acid dichromate to give acetone molecule.
The most common properties of isopropanol are given below in the table,
| Properties | |
| IUPAC name | Propan-2-ol |
| Chemical formula | C3H8O |
| Molar mass | 60.096 g/mol |
| Appearance | Colorless liquid with an alcoholic smell |
| Density | 0.786 g/cm3 |
| Melting point | -89 °C |
| Boiling point | 82.6 °C |
| Solubility | Miscible or soluble in water, benzene, chloroform, ethanol, ether, glycerin, and acetone |
| Dipole moment | 1.66 D (gas phase) |
Isopropanol may be oxidized to give a ketone with the same number of carbon atoms as its original alcohol. The oxidizing agents used for the oxidation of isopropanol are acid dichromate or alkaline potassium permanganate.
(CH3)2CHOH → (CH3)2CO + H2
Strongly electropositive metals such as potassium, sodium, magnesium, and aluminum liberated hydrogen from isopropanol to form alkoxides.
For example, sodium reacts with isopropanol to form sodium isopropoxide.
2 (CH3)2CHOH + 2 Na → 2 (CH3)2CHO–Na+ + H2
Uses of Isopropyl Alcohol
- It is used to manufacture various types of organic compounds such as acetone, glycerol, isopropyl acetate, etc.
- A small amount of isopropanol is used for the production of hydrogen peroxide.
- It is a very good solvent. Isopropyl alcohol is used for the production of various household and commercial products such as rubbing alcohol, antifreeze, disinfectants, cleaning solutions, skin and hair products, and hand sanitizers.
The most common individual uses of isopropanol are given below,
Cleaning
It is commonly used for cleaning various types of electronic devices such as audio or video tape heads, DVD and other optical discs, lenses, computers, laptops, IC packages, etc. Isopropyl alcohol is the second most commonly used alcohol after ethanol which is used in cleaning electronics or non-electronic materials or devices.
Isopropanol vapor is flammable and denser than air. Therefore, for safe use, it should be kept away from heat and open flame.
Solvent
Isopropyl alcohol can dissolve a wide range of non-polar chemical compounds. It is a good solvent for ethyl cellulose, polyvinyl butyral, essential and other oils, alkaloids, gums, resins, etc.
Medical Uses
In medicine or healthcare, isopropyl alcohol is used mainly in rubbing alcohol, disinfectants, cleaning solutions, skin and hair products, and hand sanitizers.
- Hand sanitizers and disinfectants that contain isopropyl alcohol are used in medicine to kill or prevent the growth of bacteria on the skin. We used 60–90 percent solutions of isopropanol and water in hand sanitizer.
- It also helps to prevent bacterial skin infections caused by minor cuts or scrapes.
- To relieve minor muscle pain, we also used a topical rub of isopropanol.
- Early days, it can be used in anesthesia. Due to toxicity and many side effects, isopropanol is prohibited to use in anesthesia.
Fuel Additives
Isopropanol or ethanol are the major components of fuel additives. We cannot use alcohol to remove water from fuels such as gasoline, petrol, or diesel. It can be solubilized water present in the fuels.
Laboratory
In chemical laboratories, it is used in many synthetic processes. In the biological laboratory, it is used mainly for the preservation of biological specimens.
It is a non-toxic alternative compared to formaldehyde and other synthetic preservatives. We use 70–99% propan-2-ol or isopropanol solutions for the preservation of biological specimens.
Toxicity of Isopropyl Alcohol
The toxicity of isopropyl alcohol and its metabolites is less than that of ethanol but considerably more than ethylene glycol or methanol. Poisoning occurs when our liver can no longer manage the amount of isopropanol in our body. Common symptoms or side effects of isopropanol poisoning may include,
- Stomach pain
- CNS depression
- Low blood pressure
- Confusion
- Nausea
- Vomiting
- Dizziness
- Slow breathing
Our bodies can handle a very small amount of isopropanol. Our kidneys can remove approximately 20 to 50 percent of isopropanol from our body. The rest quantities of isopropyl alcohol or isopropanol have been broken down into acetone by enzyme dehydrogenases and filtered out through your lungs or kidneys.