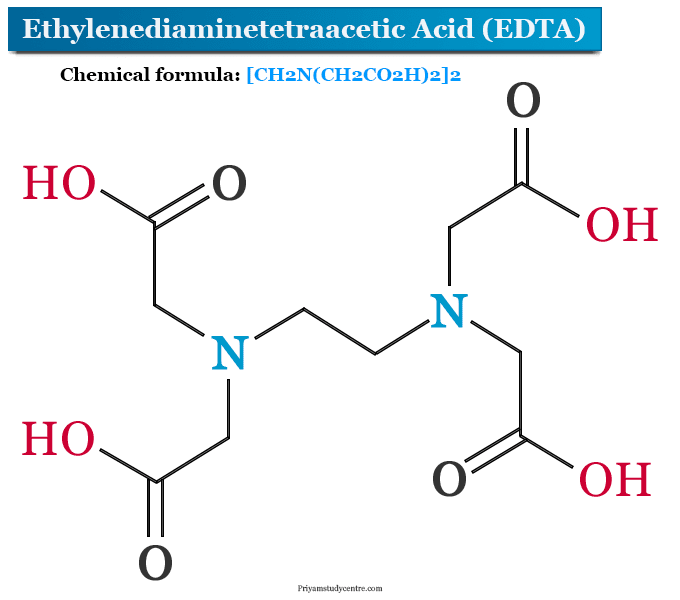
Ethylenediaminetetraacetic Acid (EDTA)
Ethylenediaminetetraacetic acid (EDTA) is an amino polycarboxylic acid commonly used as a chelating or sequestering agent with the chemical formula [CH2N(CH2CO2H)2]2. In the manufacturing industry, EDTA uses widely to improve the stability of some medicinal products, textile products, agricultural products, and cosmetics. It is readily bound to several metal ions such as iron, copper, calcium, etc. Ethylenediaminetetraacetic acid is a white, water soluble solid used widely for the determination of calcium and magnesium in hard water. Ethylenediaminetetraacetic acid forms six-membered chelate rings with a large number of metals. EDTA also formed several salts such as disodium EDTA, sodium calcium edetate, and tetrasodium EDTA. The structure of EDTA is given below the picture,
In complexometric titrations, we use disodium EDTA salt for chelate formation which behaves like a quadridentate chelating agent.
Ethylenediaminetetraacetic Acid Structure
Ethylenediaminetetraacetic acid (commonly named EDTA) is a tetraprotic acid. The structure of ethylenediaminetetraacetic acid contains ethylene diamine where all the hydrogen atoms from the amine group are replaced by four acetic acid groups. Therefore, ethylenediaminetetraacetic acid consists of 2 amino groups and four carboxyl groups in its structure.
EDTA Chelate
Ethylenediaminetetraacetic acid is a hexadentate chelating agent which is commercially available as its disodium salt. It ionizes in water to form H2Y−2. It forms a chelating complex with most of the metal ions by releasing H+ ions.
Na2H2Y → H2Y−2 + 2Na+
The formation of a cheating complex with EDTA depends upon the pH of the medium. Each metal ion forms a complex with the chelating agent at a definite pH. The structure of EDTA chelate complexes with metal ions is given below the picture,
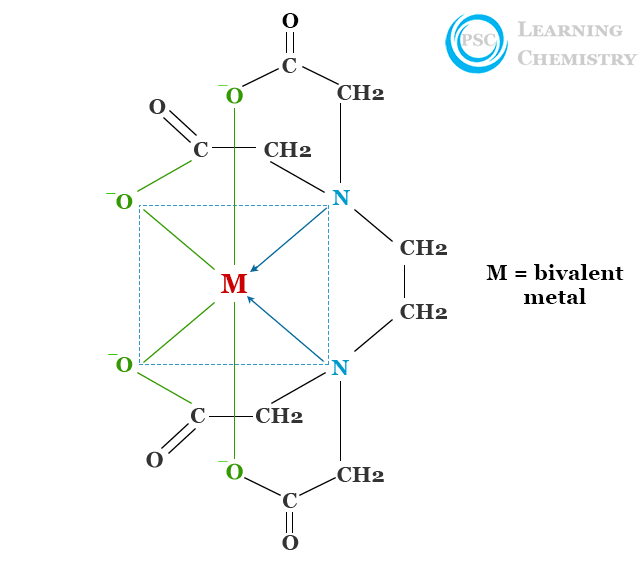
Only one metal ion is added to an ethylenediaminetetraacetic acid ion due to its hexadentate chelating character. Normally, divalent metal ions can be titrated with ethylenediaminetetraacetic acid in a basic or very weak acid medium but for trivalent or tetravalent ions, an acidic medium is required.
Synthesis
Ethylenediaminetetraacetic acid was first synthesized from ethylenediamine and chloroacetic acid in 1935 by Ferdinand Münz.
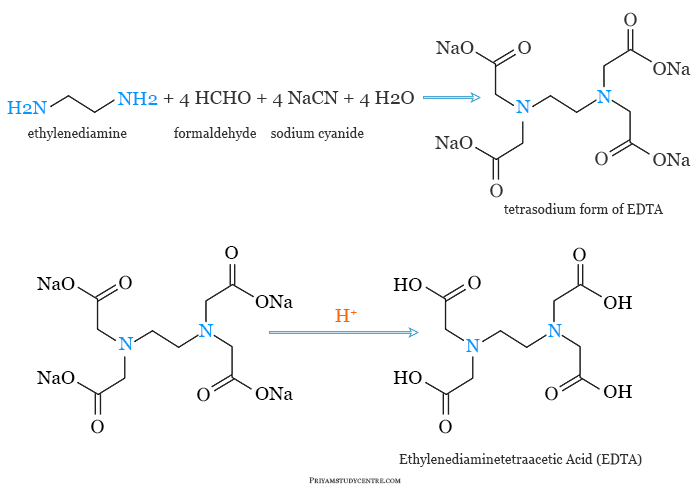
Today a high amount of ethylenediaminetetraacetic acid can be produced by the reactions of ethylenediamine (1,2-diaminoethane), formaldehyde, and sodium cyanide. The tetrasodium form of EDTA can be converted to acidic by reactions with hydrochloric acid.
EDTA Uses
EDTA (full form ethylenediaminetetraacetic acid) uses mainly as a chelating agent or sequestering agent in different laboratory processes and chemical industries.
- In the textile industry, it is used for modifying dye color by removing metal impurities from dye products.
- In the pulp and paper industry, it is used in chlorine-free bleaching.
- It is added to some food products for the preservation or stabilization of food colour.
Many other individual applications of ethylenediaminetetraacetic acid are given below,
Uses in Water Treatment
- Water treatment is a process by which we improve the quality of water for its specific uses. Hard water does a lot of harm to industrial boilers, laundry, and other equipment. Therefore, ethylenediaminetetraacetic acid can be used for removing such salts from water which we used in laundry and many chemical processes.
- Total hardness due to dissolved calcium and calcium magnesium salts can be determined by EDTA titration.
Laboratory Applications
- In chemistry, ethylenediaminetetraacetic acid is mainly used as a chelating or sequestering agent. It forms a stable water soluble metal chelate.
- It is widely used for the determination of different metal ions by complexometric titration. For example, total calcium and magnesium can be determined by EDTA titration by using the Eriochrome Black T (EBT) indicator.
- In analytical chemistry, EDTA is used in the separation of lanthanide metals by ion-exchange chromatography.
- EDTA is a chelating agent or sequestrant. In biochemistry and molecular biology, EDTA can be used to minimize metal ion contamination and prevent enzymes activity. Disodium EDTA is used separation of DNA and protein by electrophoresis.
Medicinal Use
- In medicine, an ethylenediaminetetraacetic acid derivative is used to bind harmful metal ions by chelation therapy. We used an intravenous injection of calcium sodium derivative of EDTA for treating mercury and lead poisoning. Similarly, it is also applicable for removing excess iron from our bodies. It is approved by the U.S. Food and Drug Administration (FDA) for treating lead or mercury poisoning.
- It is also used to remove high levels of calcium from our bodies. The disodium form of EDTA can be used to clear calcium deposits in the eye to improve our eyesight.
- Some medicines can use ethylenediaminetetraacetic acid for treating several health diseases such as diabetes, peripheral vascular disease, Alzheimer’s disease, and heart disease.
- It is an anticoagulant for blood samples. Therefore, ethylenediaminetetraacetic acid is widely used for the analysis of blood samples.
Cosmetics
EDTA can be used in several cosmetics products such as shampoos, cleansers, and other beauty products. Due to sequestering or chelating properties, ethylenediaminetetraacetic acid may improve the stability of such products in the air.
Complexometric Titration with EDTA
The sequestering or chelating effect of ethylenediaminetetraacetic acid has led to the development of some most powerful volumetric methods of analysis. It rapidly and strongly complexes several metal ions such as calcium, magnesium, iron, copper, etc.
A common example of a typical EDTA titration is the determination of calcium and magnesium in hard water. The total calcium and magnesium ion are found by titrating pH 10 with the Eriochrome-Black-T (EBT) indicator. Then magnesium is precipitated as Mg(OH)2 at a pH range of 7 to 11 and calcium is triturated with ethylenediaminetetraacetic acid with a mureide indicator. Free copper should be masked with hydrogen sulfide.
There are several advantages of EDTA titration in comparison with other kinds of titrations.
- It is stable, soluble, and forms a definite complex with a metal ion.
- It can be made more selective by the control of pH.
- Stable complexes are formed at low pH to avoid hydrolysis.
- Disodium salt of EDTA is a primary standard. Therefore, no need for further standardization.
- The equivalence point is readily reached in such types of titration. Therefore. it can be used for the determination of several types of metal ions in semi-micro scale operations.
Side Effects
- It has several side effects on human health which depend on various factors such as dosage, renal function, and frequency of administration. Renal toxicity is the most common side effect of EDTA.
- It can also cause zinc deficiency in smaller children due to the strong affinity of calcium EDTA towards zinc metal.
- It has cytotoxic and weakly genotoxic properties which affect animals in reproduction and development.
- Other most common side effects of ethylenediaminetetraacetic acid or EDTA are fever, nausea, vomiting, chills, fatigue, arthralgia, hypotension, arrhythmias, acute tubular necrosis, proteinuria, tremors, headache, cheilosis, bone marrow depression, anemia, and hypercalcemia.