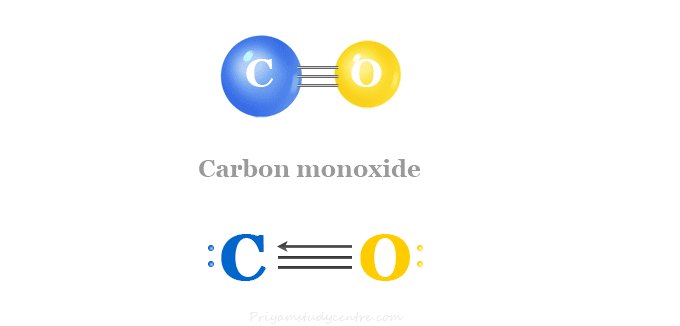
Carbon Monoxide (CO)
Carbon monoxide (chemical formula CO) is a colurless, odourless, poisonous gas produced by burning carbon in insufficient oxygen. In the laboratory, carbon monoxide is produced by dehydrating formic acid with concentrated sulfuric acid. Carbon monoxide is a very weak Lewis base but it acts as a strong ligand to transition metals. Nickel forms volatile Ni(CO)4 at ordinary temperature. However, many other transition metals form a wide variety of carbonyls under different conditions. The synthesis gas (mixture of CO and hydrogen) is used for the production of various organic compounds.
Bonding in Carbon Monoxide
Carbon monoxide has a total of ten electrons in the outer orbitals. On the hybridization model, we assumed both carbon and oxygen to have sp hybridized.
| Carbon | ↑↓ | ↑ | ↑ | hybridized | ↑↓ | ↑ | ↑ | ||
| 2s | 2px | 2py | 2pz | 2spx | 2spx | py | pz |
| Oxygen | ↑↓ | ↑↓ | ↑ | ↑ | hybridized | ↑↓ | ↑ | ↑ | ↑↓ |
| 2s | 2px | 2py | 2pz | 2spx | 2spx | py | pz |
The pairs of electrons in sp orbitals of carbon and oxygen make the lone pairs of CO. The sp orbitals of carbon and oxygen overlap to form the σ covalent bond in CO. The two py orbitals overlap to form one π bond.
Another electron pair on the pz orbital of oxygen overlaps with the empty pz orbital of carbon to form the second π bond. The last one is the coordinate type bond.
Molecular Orbital Diagram of Carbon Monoxide
The chemical bonding in carbon monoxide is best represented by the molecular orbital diagram given below the picture,
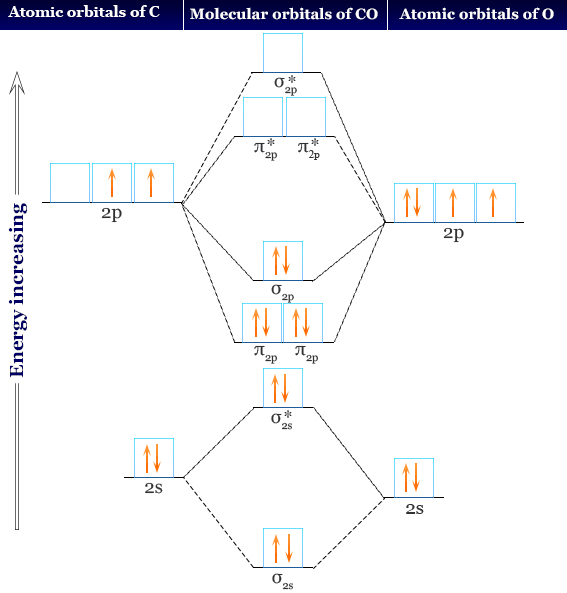
- On the molecular orbital model of carbon monoxide, the two spx hybrid orbitals of oxygen and carbon combine to give two molecular orbitals. One is low energy sigma bonding type that contains two electrons and the other is high energy sigma antibonding type.
- The two py orbitals will combine to give two π-type molecular orbitals. One is the bonding type being occupied by two electrons and the other is the antibonding type.
- Similarly, the two px orbitals of carbon and oxygen will give two molecular orbitals. The bonding type orbitals contain two electrons but the antibonding type is empty.
All the bonding molecular orbital in CO are filled. There are three antibonding orbitals of which spx is much higher energy. This particular molecular orbital is placed along the C-O bond axis and is not accessible for back donation.
The other two molecular orbitals are available to metal for back donation. Since oxygen is more electronegative than carbon. Therefore, the carbon lone pair functions as a donner.
Properties of Carbon Monoxide
Carbon monoxide is an electron-deficient molecule and has π-acceptor properties. It is an ideal ligand for a low oxidation number or state of metal. CO is sparingly soluble in water and burns in air to form carbon dioxide with a blue flame.
It is extraordinarily poisonous. CO combines with the hemoglobin of blood. Therefore, it renders to carry oxygen by red blood cells. Some basic physical properties of carbon monoxide are given below in the table,
| Properties of carbon monoxide | ||
| Chemical formula | CO | |
| Appearance | Colouless gas | |
| Molar mass | 28.010 g/mol | |
| Density | Liquid | Gas |
| 789 kg/m3 | 1.250 kg/m3 at 0° C and 1 atm pressure | |
| Melting point | −205.02 °C | |
| Boiling point | −191.5 °C | |
| Solubility in water | 27.5 mg/L at 25°C | |
| Dipole moment | 0.122 D | |
| Heat capacity | 29.1 J mol−1 K−1 | |
Production Process
Controlled oxidation or catalytic steam reforming of methane or natural gas gives CO.
The gasification of coal with oxygen at 1500 °C gives carbon monoxide.
C + H2O → CO + H2
CO2 formed by this process may be removed by washing monoethanolamine. Sulfur compounds can be removed before the synthetic use of CO.
Chemical Reaction
When burned in the air, it produces a blue flame of carbon dioxide (CO2).
2 CO + O2 → 2CO2 (ΔH0 = −565 kJ mol−1)
Carbon monoxide generally reduces ammoniacal silver nitrate salt to silver metal and palladium chloride salt to palladium metal.
PdCl2 + H2O + CO → Pd + 2 HCl + CO2
Alkali metals in liquid ammonia react with CO to produce colorless crystalline solid salts containing [OC≡CO]−2 ion.
2 Na + 2 CO → NaOC≡CONa
It reduces many metal oxides. For example, I2O5 can be reduced by CO to form iodine. This reaction may be used for the detection of CO.
I2O5 + 5 CO → I2 + 5 CO2
Uses of Carbon Monoxide
- Carbon monoxide generally uses in industry for the production of water gas and synthesis gas. Pure hydrogen may be obtained by the water gas shift (WGS) reaction.
CO + H2O → CO2 + H2 (ΔH0 = −41.2 kJ mol−1) - Carbon monoxide is a major constituent of gaseous fuels including coal gas that is used as a domestic fuel through pipeline supply.
- It gives a variety of products under varying conditions when catalytically reduced by hydrogen
- Heterogeneous reduction of CO by hydrogen catalyzed by metal gives liquid hydrocarbon. The liquid hydrocarbon is used as a substitute for natural petroleum. The process is called Fischer-Tropsch synthesis. Through this process, one may obtain a variety of products like methane, waxy hydrocarbon, methanol, and higher alcohol.
- It is used in the packaging of food products like fish, beef, etc.
- It is also used in infrared lasers and removes rust from the metal surface.
- Carbon monoxide and hydrogen are added to olefinic bonds to produce aldehydes and alcohols.