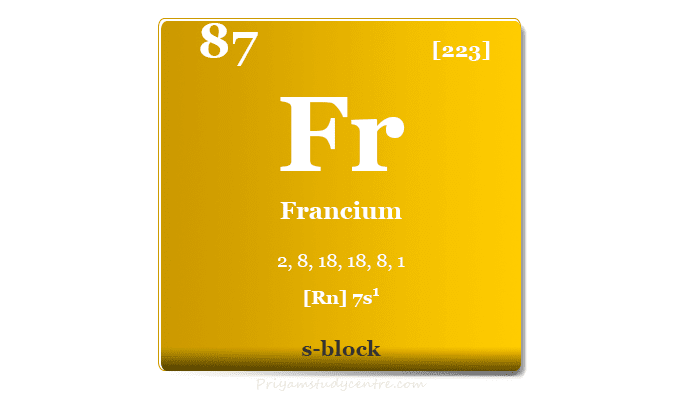
Francium Element
Francium is a chemical element of the alkali metal family in the periodic table with the symbol Fr and atomic number 87. It is a soft, low, melting, silvery white metal placed along with lithium, sodium, potassium, rubidium, and caesium. The electronic configuration of a francium atom is [Rn] 7s1 and it was the last member of the alkali metal family that was missing for a long period. It is a very rare chemical element and not found outside the laboratory while a trace amount of francium is found in uranium and thorium ores.
Francium cannot be used for any commercial purposes. It is used only for research purposes in the field of chemistry. The study of Fr provides useful information about the atomic structure such as elementary particles and the energy levels of an atom.
The most stable isotope, Fr-223 has high radioactivity and a very small half-life (22 minutes). The isotopes of francium decay quickly to form astatine, radium, and radon.
Where is Francium Found?
A trace amount of francium isotopes is found in uranium minerals. In the earth’s crust, about 30 g of francium is found at any given time. The terrestrial abundance of metal is estimated at about 2 × 10−18 ppm.
It may be obtained by the neutron bombardment of radium in a nuclear reactor.
Isotopes
Francium has 34 known isotopes with atomic masses ranging from 199 to 232. Fr-223 and Fr-221 are the only naturally occurring isotopes of the alkali metal.
- Fr-223 is the most stable isotope found in the uranium-235 decay series. The half-life of Fr-223 is 21.8 minutes. It formed Ra-223 by beta decay and At-219 by alpha decay.
87Fr223 → 88Ra223 + −1e0 - Fr-221 is another isotope found in nature. The half-life of this isotope is 4.8 minutes. Francium-221 is the ninth product of the neptunium decay series.
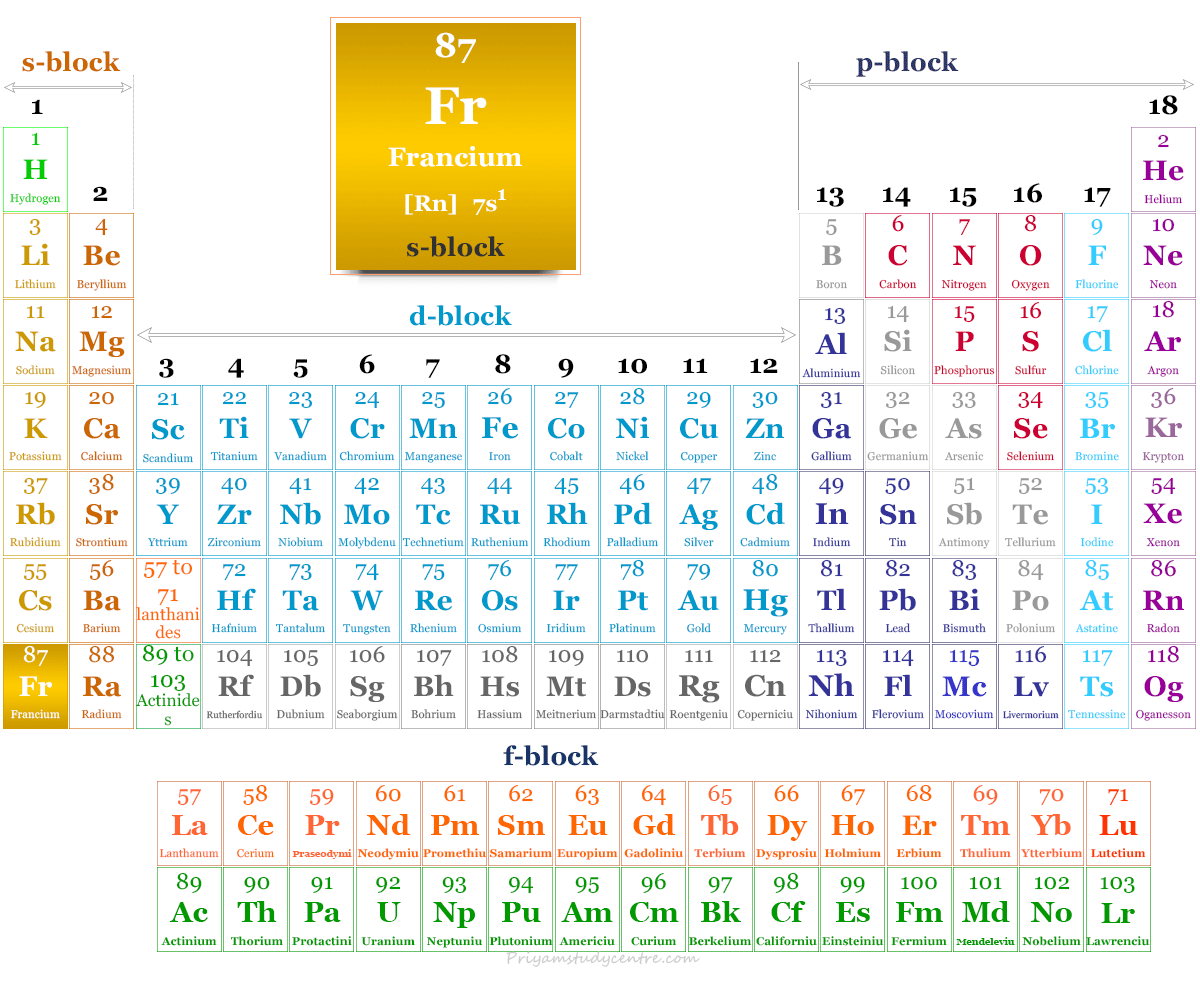
Francium on the Periodic Table
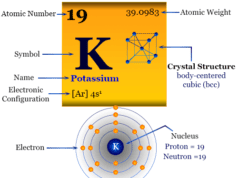
It is found in group 1 and period 7 of the periodic table. Francium is an alkali metal that lies along with Li, Na, K, Rb, and Cs.
Properties
Francium is the most usable naturally occurring radioactive element with a very small half-life. The half-life of the longest live isotope is 22 minutes. It is considered to be the second rarest metal on the earth’s crust after astatine.
The valence shell electronic configuration suggests that the chemical and physical properties of francium are similar to the alkali metal caesium.
| Francium | |
| Symbol | Fr |
| Discovery | Marguerite Perey in 1939 |
| Name derived from | named from the country France |
| Common isotope | 87Fr233 |
| Oxidation states | +1 |
| CAS number | 7440-73-5 |
| Periodic properties | |
| Atomic number | 87 |
| Relative atomic mass | [223] |
| Electron per cell | 2, 8, 18, 32, 18, 8, 1 |
| Electronic Configuration | [Rn] 7s1 |
| Block | s-block |
| Group | 1 |
| Period | 7 |
| Physical properties | |
| State at 20 °C | Solid |
| Melting point | 21 °C, 70 °F, 294 K |
| Boiling point | 650 °C, 1202 °F, 923 K |
| Crystal structure | body-centered cubic (bcc) |
| Density | 2.48 g/cm3 (estimated) |
| Electrical resistivity | 3 µΩ m |
| Atomic properties | |
| Atomic radius (non-bonded) | 3.48 Å |
| Covalent radius | 2.42 Å |
| Electronegativity | 0.7(Pauling scale) |
| Electron affinity | 44.38 kJ mol−1 |
| Ionization energy (kJ/mol) | 1st |
| 392.96 | |
Chemical Compounds
It is very rare in our earth’s environment. It may not be found outside the laboratory. Therefore, naturally occurring chemical compounds of metal are rare in nature.
Francium is the most electropositive element in the periodic table. Coprecipitation studies revealed the similarities between Fr and Cs.
It is carried down completely on the crystals of Cs2PtCl6, Cs2SnCl6, and Cs2[Co(NO2)6] to form respective salts. It can be precipitated from hydrochloric acid solution by silicotungstic acid.
Production Process
Francium can be produced by a nuclear fusion reaction. It can be synthesized in a linear accelerator by the bombardment of a beam of oxygen-18 atoms on a gold-197 target.
Depending on the energy of the oxygen beam, the nuclear reaction can produce francium isotopes like 209Fr, 210Fr, and 211Fr.
79Au197 + 8O18 → 87Fr209 + 6 0n1
79Au197 + 8O18 → 210Fr + 5 0n1
79Au197 + 8O18 → 211Fr + 4 0n1
It is also obtained by the neutron bombardment of radium in a nuclear reactor. It may be made by bombarding thorium with protons.
Uses of Francium
Francium is a highly radioactive and rare metal. The half-life of the most stable isotope Fr-223 is only 22 minutes. Therefore, it cannot be used for any commercial purposes.
It may be used in the field of chemistry to generate information about atomic structure. The spectroscopic experiments with francium provide useful information about subatomic particles and the energy levels of an atom.