Gold Metal
Gold is a chemical element or yellow precious d-block metal of Group 11 or 1B of the periodic table with the symbol Au and atomic number 79. It is chemically unreactive, extremely malleable, ductile, high density (19.32 gm cm−3) golden-yellow metal. The metal is also a good conductor of heat and electricity. Gold metal has been used mostly from ancient history for jewelry or coinage making. The name gold metal is derived from the old English word geolu means yellow and from the Latin word aurum. The metals gold, silver, and copper occur in nature in a native form with very similar chemical and physical properties in chemistry. Copper, silver, and gold constitute the family of coinage or currency metals. The purity of the metal is generally expressed in carats, pure gold is 24 carats and the common alloyed with copper is 22 carats.
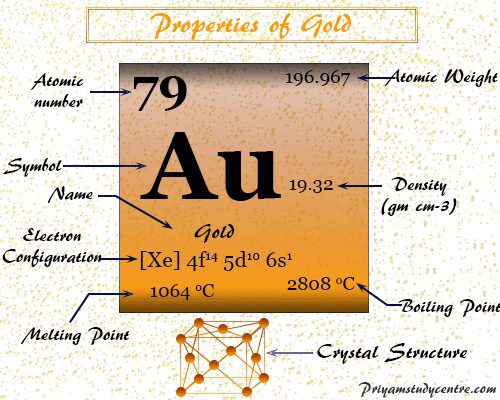
Properties of Gold
In learning chemistry, the characteristic golden yellow color arises due to the absorption in the UV light from blue regions of the electromagnetic spectrum corresponding to the excitation of electrons from the filled d-band to the s-p conduction band.
The metal from the face-centered (fcc) crystal lattice in the solid form.
| Gold |
||
| Symbol | Au | |
| Discovery | approx 3000BC | |
| Name derived from | The old English word geolu means yellow and from the Latin word aurum | |
| Common isotope | 79Au197 | |
| CAS number | 7440-57-5 | |
| Periodic properties |
||
| Atomic number | 79 | |
| Atomic weight | 196.967 | |
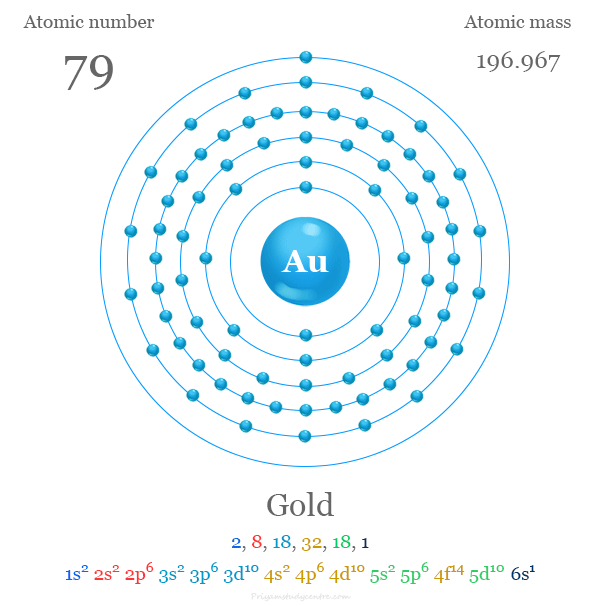
| Electron per cell | 2, 8, 18, 32, 18, 1 | |
| Electronic configuration | [Xe] 4f14 5d10 6s1 | |
| Group | 11 | |
| Period | 6 | |
| Block | d-block | |
| Physical properties |
||
| State at 20 °C | Solid | |
| Meting point | 1064 °C | |
| Boiling point | 2808 °C | |
| Density | 19.32 g/cm3 | |
| Molar heat capacity | 25.418 J mol−1 K−1 | |
| Crystal structure | face-centered cubic (fcc) | |
| Electrical resistivity | 22.14 nΩ m | |
| Chemical properties | ||
| Atomic radius (non-bonded) | 2.14 Å | |
| Covalent radius | 1.30 Å | |
| Oxidation states | 5,4,3,2, 1,-1 | |
| Electronegativity | 2.54 (Pauling scale) | |
| Electron affinity | ||
| Ionization energy (kJ mol−1) | 1st | 2nd |
| 890.1 | 1980 | |
Electron Configuration of Gold
The 79 electrons of the Au atom are distributed in different orbitals to give the following electronic configuration given in the picture,
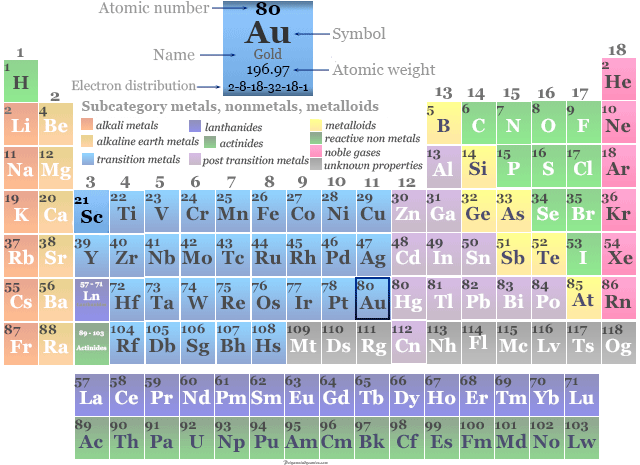
Gold on the Periodic Table
Gold is placed in group 11 on the periodic table with a d-block or transition metal.
The valence shell electronic configuration of Au [Xe] 4f14 5d10 6s1 with filled d-orbital. It is placed with transition metal due to the presence of vacant d-orbital in the +3 oxidation number or state.
Where is Gold Found?
Native Au is found in nature in two forms,
- Reef-Au: deposit or dispersed fine particles in quartz veins in deep mines.
- Alluvial-Au: primary deposit by geological action and redeposited in alluvial sands gravel as fine grains.
The combined form of gold is found mostly in a few minerals like calaverite, AuTe2, and sylvanite, (Ag, Au)Te2.
It is also found in sea water to the extent of 10−3 ppm but no extraction methods were developed to gain the metal from sea water.
Gold Production by Country
The top 10 commercial gold producer countries in the World according to the 2019 report given below table,
| Country | Gold production (tonnes) |
| China | 383.2 |
| Russia | 329.5 |
| Australia | 325.1 |
| United States | 200.2 |
| Canada | 182.9 |
| Peru | 143.3 |
| Ghana | 142.4 |
| South Africa | 118.4 |
| Mexico | 111.4 |
| Brazil | 106.9 |
Gold Processing Steps
Gold is processed mainly by the cyanide and amalgamation process.
Cyanide Gold Extraction Process
The cyanide process is going through the following steps,
- The finely powdered rocks were ignited with dilute sodium cyanide (NaCN) solution and lime in the presence of air for oxidation.
4 Au + 8 NaCN + 2 H2O + O2 → 4 Na[Au(CN)2] + 4 NaOH - The solution is filtered and Au is deposited from the filtrate by zinc shavings.
Zn + 2 Na[Au(CN)2] → 2 Au + Na2[Zn(CN)4] - Zinc generally is dissolved by dilute sulfuric acid and the dried residue is melted with borax.
- Crude gold contains copper, silver, and lead. Lead is removed by cupellation.
- Copper is removed by oxidative fusion of borax but silver may be removed by boiling with concentrated sulfuric acid.
- However, electrolysis is the best method for refining Au.
Amalgamation Process
The amalgamation process was also used to separate native gold from alluvial sand and gravel deposits. The finely crusted rocks amalgamated with mercury with the stream of water over copper plates. The amalgam is scary and mercury is distilled out in iron retorts.
Interesting Facts
- The first ionization energy of Ag is the lowest among the noble metals family. The sum of the first and second ionization energy of Cu is the lowest. However, the sum of the first, second, and third ionization energy of Au is the lowest. The fact suggests the common oxidation state of Cu, Ag, and Au.
- For gold, atomization, ionization, and hydration energy favor the formation of Au(III) in aqueous solutions.
Chemical Compounds
The chemically unreactive gold is the noblest transition metal on the periodic table. Therefore, it does not attract oxygen, or sulfur, or readily reacts with halogens like fluorine, chlorine, bromine, and iodine molecule.
It will be dissolved in cyanide solution in the presence of hydrogen peroxide or aqua regia (a mixture of concentrated nitric acid and hydrochloric acid).
Gold Halide
Although the small size but no simple monovalent cation of gold can exist, most of the compounds are formed by covalent bonding or by the form of complexes.
The simple compound of gold (I) is restricted to chlorine, bromine, and iodine only. AuCl and AuBr are prepared by controlled thermal decomposition of respective halides.
AuI is prepared by heating the metal with iodine solution or adding iodine solution to AuCl3 solution.
Gold Fluoride
The orange crystalline solid of Au (III) fluoride is made by the action of fluorine on Au2Cl6 at 300 °C.
AuF7 and AuF5 are examples of Au compounds that show a higher than +3 oxidation state formed by covalent chemical bonding.
Oxides
The oxide, Au2O obtained by dehydrating AuOH with alkali metals and sulfur dioxide. Au (III) is the most common oxidation state of gold offering oxide like Au2O3.
Amorphous brown Au2O3, 2H2O is precipitated by alkali from the solution containing AuCl4−. However, the anhydrous oxide is obtained by drying the hydrated compounds over P4O10 followed by careful heating.
Fulminating Gold
It is an olive green explosive powder obtained by careful digestion of Au2O3 or its hydrate with ammonia. The dry powder explodes with a flash on striking or heating. The probable formula of fulminating Au is HN=Au-NH2, 1.5 H2O.
Complex Compound
+2 oxidation state is unfavorable in comparison to Au (I) and Au (II) and few complexes of Au( II) have been claimed.
For example, tetra n-butylammonium salt of the bis (maleonitriledithiolato) aurate(II).
Au2S3 is produced when passing hydrogen sulfide over dry LiAuCl4, 2H2O at 10 °C. The lithium chloride is separated by extracting with alcohol and dried at 70 °C.
Uses of Gold
- Gold and its compounds are extensively used in medicine in old age Ayurveda as well as in modern Allopathy. It is used particularly for the treatment of arthritis.
- Because of its bright color and low chemical reactivity, gold was the first metal that attracted the human eye to prepare coins, different types of craft jewelry, and decorative objects.
- Due to their unique qualities, the materials are universally accepted in goods and services in the form of coins or bullion to affect the currency of the world.
- The modern currency is paper-based currency but gold plays a key role dominate the currency of the country.
- It is accepted by all nations as a medium of international payment technique.
- A purple powder containing a colloid of Au is absorbed into the hydrated tin (II) oxide. It is used mostly for making ruby glass. The purple powder is prepared generally by reducing Au (II) chloride with SnCl2.
- Due to electrical conductivity, thermal conductivity, and unreactive properties, we use a large amount of gold for industrial purposes in electrical or electronic engineering.
- It is used for making terminals, plating contacts, printed circuits, and semiconductors.
What is 24k Gold?
Pure gold is 24 carats containing 24 parts of Au by weight and 2 parts of other metals in weight
It is alloyed with other metals due to its increased softness and brightness for easy use for jewelry, gold ware, and coin making. The alloyed metals are silver, copper, zinc, platinum, and palladium.
The silver alloyed gold is used to make coins or gold ware but the platinum or palladium alloyed metal is commonly used for making craft jewelry.