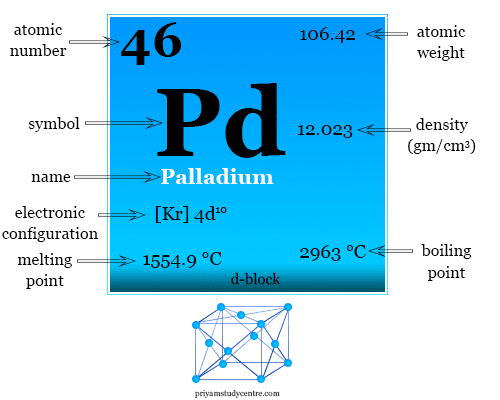
What is palladium?
Palladium is a chemical element or silvery white, lustrous, malleable, high density metal of group-10 of the periodic table with atomic number 46 and symbol Pd. The elements namely ruthenium, rhodium, palladium, osmium, iridium, and platinum are called platinum metals.
Chemically, Palladium metal is inert to attack by mineral acids and air or water under ordinary conditions. Almost all compounds decompose to yield Pd when heated. All these elements are rare in the earth’s crust. The element palladium is found together with other noble metals like copper, silver, and gold.
Palladium discovery
Palladium is the second platinum metal discovered in 1803 by English chemist W Wollaston. It was isolated and identified during the investigation of crude platinum. He called the metal after the asteroid Pallas discover a year earlier. He named it after the Greek goddess of wisdom.
Palladium properties
Chemically, all platinum metals (Ru, Rh, Pd, Os, Ir, Pt) are relatively noble. They are relatively inert to attack by mineral acids and water under ordinary conditions. Os and Pd dissolve slowly in oxidizing acids like sulfuric acid or nitric acid.
It has the remarkable properties of absorbing molecular hydrogen in large volumes. Electronic configuration and some physical and chemical properties of palladium are given below the table,
| Palladium properties |
||
| Atomic number | 46 | |
| Electronic configuration | [Kr] 4d10 | |
| Atomic weight | 106.42 | |
| Melting point | 1554.9 °C | |
| Boiling point | 2963 °C | |
| Density | 12.023 g/cm3 | |
| Molar heat capacity | 25.98 J mol−1 K−1 | |
| Electrical resistivity | 105.4 nΩ·m | |
| Crystal structure | face-centered cubic (fcc) | |
| Group | group-10 | |
| Period | period-5 | |
| Block | d-block | |
| Chemical properties | ||
| Oxidation number | +2, +4 | |
| Electronegativity | Pauling scale: 2.20 | |
| Ionization energy (kJ/mol) |
1st | 2nd |
| 804.4 | 1870 | |
From group-10 elements like Ni, Pd, and Pd, the ionization energy increases with the increasing atomic number. Due to lanthanide construction, the atomic radius of Pd and Pt are the same.
Electron configuration of palladium
The 46 electrons of the Pd atom are distributed in different energy levels to show the following electronic configuration given below the picture,
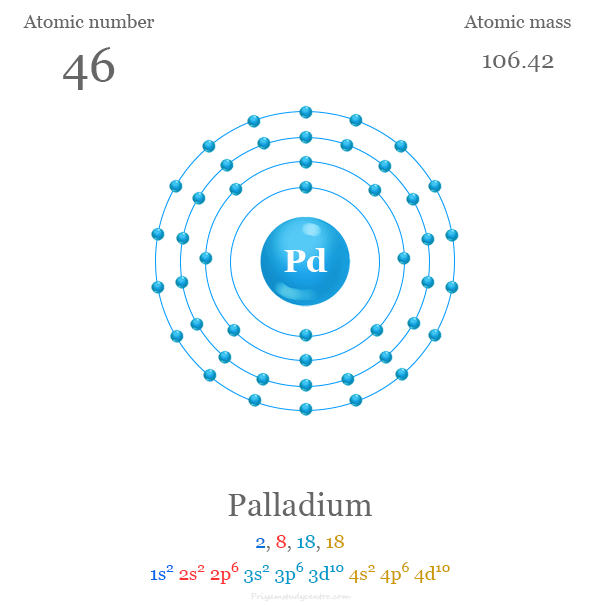
Palladium in the periodic table
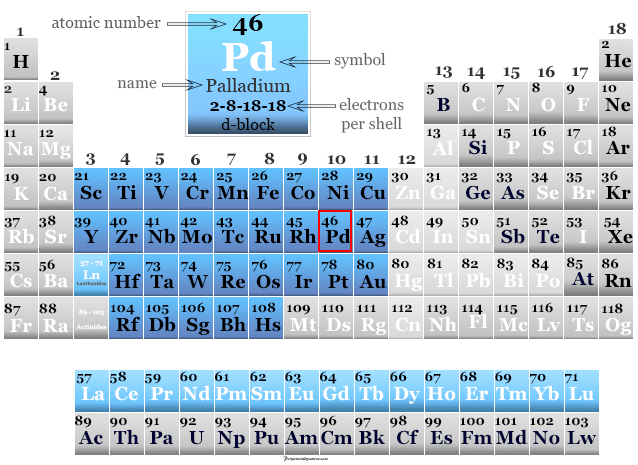
Palladium is placed in group-10 and period-5 of the periodic table with d-block elements or transition metals. The valence shell electronic configuration of platinum is [Kr] 4d10. It has many similarities with group member platinum. +2 and +4 oxidation number or state is the most common for both metals.
Where is palladium found?
The chief sources of palladium are South Africa, Canada, Russia, Brazil, Columbia, etc. It is rare in the earth’s crust and found at about 0.015 ppm. Copper or nickel sulfide ores of the Sudbury district of Ontario state (Canada) have to contain the highest amount of Pd. Huge quantities of palladium are extracted as a by-product from copper or nickel sulfide.
Isotopes
Naturally occurring palladium contains seven isotopes. The radioactive isotope 107Pd is the most stable (half-life 6.5 million years). Eighteen other radioactive isotopes are obtained by different types of nuclear reactions. These isotopes have a very short half-life.
Production process
Platinum metals are mostly obtained from copper or nickel ores. In the production of copper or nickel, the anode slime contains about 4 percent of platinum metals.
Separation and isolation of individual platinum metals are based on their properties towards acids or the differences in solubility of their complexes.
- In the treatment of anode slime by aqua regia, the Pd together with Au and other platinum metals are soluble to form chloro complexes. Palladium from the complex like H2PdCl4.
- From the solution, gold is precipitated by FeSO4.
- The solution containing H2PdCl4 left after the precipitation of platinum is treated with excess aqueous ammonia followed by HCl. The precipitate of [Pd(NH3)2]Cl2 is purified by precipitation and ignited to obtain palladium.
- Palladium element is obtained in the form of a powder sponge. It is made compact by metallurgical technique.
Uses of Palladium
- Palladium has remarkable properties to absorbed hydrogen gas which remains in an active state. Because of its ability to absorb hydrogen, it is used as a chemical catalyst in different types of industrial and laboratory reactions.
- It is used in various important catalytic reactions like the hydrogenation of acetylene to ethylene.
- It is used for the production of hydrogen peroxide by oxidation reduction of 2-ethylanthraquinol and 2-ethyl anthraquinone.
- For making multi-layer ceramic capacitors, we used a huge quantity of Pd-Ag alloy.
- It is also used as a different type of electronic component making materials or soldering materials.
- Pd membranes are used for the production of pure hydrogen gas due to the absorbance properties of palladium.
- Pd hydrogen electrodes are widely used in various electrochemical cells.
- It is not blackened by hydrogen sulfide (H2S). Therefore, it is used for making jewelry.
Chemical compounds
+4 and +2 oxidation states are the most common oxidation states for the element palladium. The highest oxidation state of Pd element is +4. PdF4 is the only tetrahalide in the +4 state. Fluorination of K2[PdCl4] gives K2PdF6. The anion PdF6−2 is thermodynamically unstable to hydrolysis.
It forms numerous complexes which are diamagnetic and mostly are square planners. Some common chemical compounds of palladium in different oxidation states are discussed below,
Palladium oxide
A black colour oxide (PdO) is prepared by heating palladium in oxygen. PdO dissociates above 875 °C and is insoluble in all acids.
A gelatinous dark yellow precipitate of the hydrous oxide PdO, xH2O may be obtained by adding alkali in aqueous Pd(NO3)2. It is soluble in acids but can not be completely dehydrated.
Palladium chloride
Pd(II) chloride has two forms, α-PdCl2 and β-PdCl2. Heating Pd metal in chlorine above 550 °C produces α-PdCl2 which is slowly transforming into β-PdCl2.
- The α-form has an infinite planner chain structure.
- But the β-PdCl2 forms an octahedral structure. The whole unit has the molecular formula Pd6Cl12 but there is no indication of Pd-Pd bonding. Palladium chloride is soluble in water and accepts chloride ions to form PdCl4−2.