Promethium Element
Promethium is a chemical element or rare earth metal in the periodic table with the symbol Pm and atomic number 61. It is a radioactive lanthanide or f-block element that is extremely rare in Earth’s crust. All the isotopes of promethium are radioactive and found naturally by the spontaneous fission of uranium-238. Promethium is the only element in the lanthanide series of the periodic table that has no stable isotopes. It is used mainly for research purposes due to the very small abundance of rare earth metal promethium. The only isotope Pm-147 is used in luminous paint, atomic batteries, and thickness-measurement devices. A very trace amount of promethium element is found in the earth’s crust. It is also identified in the spectrum of the star HR465 in Andromeda.
In 1945, USA chemists Jacob Marinsky, Lawrence Glendenin, and Charles Coryell isolated the radioactive isotopes 147Pm and 149Pm from uranium fission products at Clinton Laboratories. The name promethium was proposed by discoverers from the word Prometheus. In Greek mythology, Prometheus means stealing fire from the Gods and giving it to humans.
Where is Promethium Found?
It is the only element in the lanthanide series of the periodic table that has no stable isotopes. Only a trace amount of promethium is found in the Earth’s crust in some uranium ores. In addition to uranium ores, the Pm spectrum may also be found in the Andromeda galaxy.
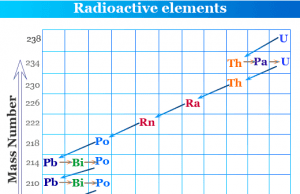
It is formed in nature as a product of spontaneous radioactive decay of uranium-238. It is extremely rare and only about 500–600 grams of naturally occurring Pm metal is found in Earth’s crust at any given time. The isotope promethium-147 is also formed by alpha decays of natural europium-151.
Properties
It belongs to the cerium group of lanthanides. Promethium adopts a double hexagonal close-packed (DHCP) crystal structure.
Chemically, promethium is very similar to the neighboring elements neodymium and samarium. The ionic radius of Pm is 110 pm. It is very similar to its neighboring neodymium (112 pm) and samarium (108 pm).
| Promethium | |||
| Symbol | Pm | ||
| Discovery | Jacob .A. Marinsky, Lawrence E. Glendenin, and Charles D. Coryell in 1945 | ||
| Name derived from | Prometheus, in Greek mythology, stole fire from the Gods and gave it to humans | ||
| Common isotopes | 61Pm145, 61Pm147 | ||
| Oxidation states | +3 | ||
| CAS number | 7440-12-2 | ||
| Periodic properties | |||
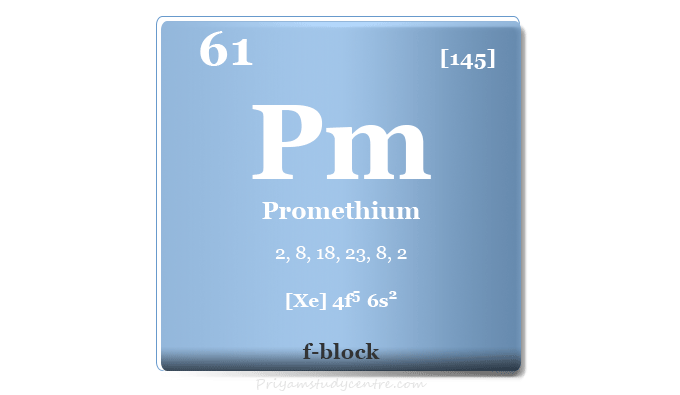
| Atomic number | 61 | ||
| Relative atomic mass | [145] | ||
| Electron per cell | 2, 8, 18, 23, 8, 2 | ||
| Electronic Configuration | [Xe] 4f5 6s2 | ||
| Block | f-block | ||
| Group | Lanthanides | ||
| Period | 6 | ||
| Physical properties | |||
| State at 20 °C | Solid | ||
| Melting point | 1042 °C, 1315 K | ||
| Boiling point | 3000 °C, 3273 K | ||
| Magnetic Ordering | paramagnetic | ||
| Crystal structure | double hexagonal close-packed (dhcp) | ||
| Density | 7.26 g/cm3 | ||
| Heat of fusion | 7.13 kJ mol−1 | ||
| Heat of vaporization | 289 kJ mol−1 | ||
| Atomic properties | |||
| Atomic radius (non-bonded) | 2.38 Å | ||
| Covalent radius | 1.86 Å | ||
| Electronegativity | Unknown | ||
| Electron affinity | Unknown | ||
| Ionization energy (kJ/mol) | 1st | 2nd | 3rd |
| 538.58 | 1051.70 | 2151.60 | |
Electron Configuration
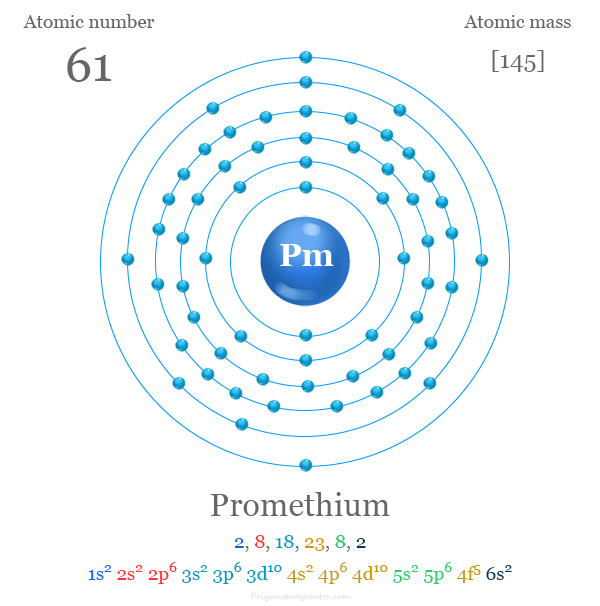
The 61 electrons of promethium are arranged to show the electron configuration of [Xe] 4f5 6s2. Like other lanthanides, Pm uses three valence electrons to show a +3 oxidation number or state. The remaining 4f electrons are strongly bound due to the higher stabilization of 4f electrons in comparison to 5d or 6s electrons.
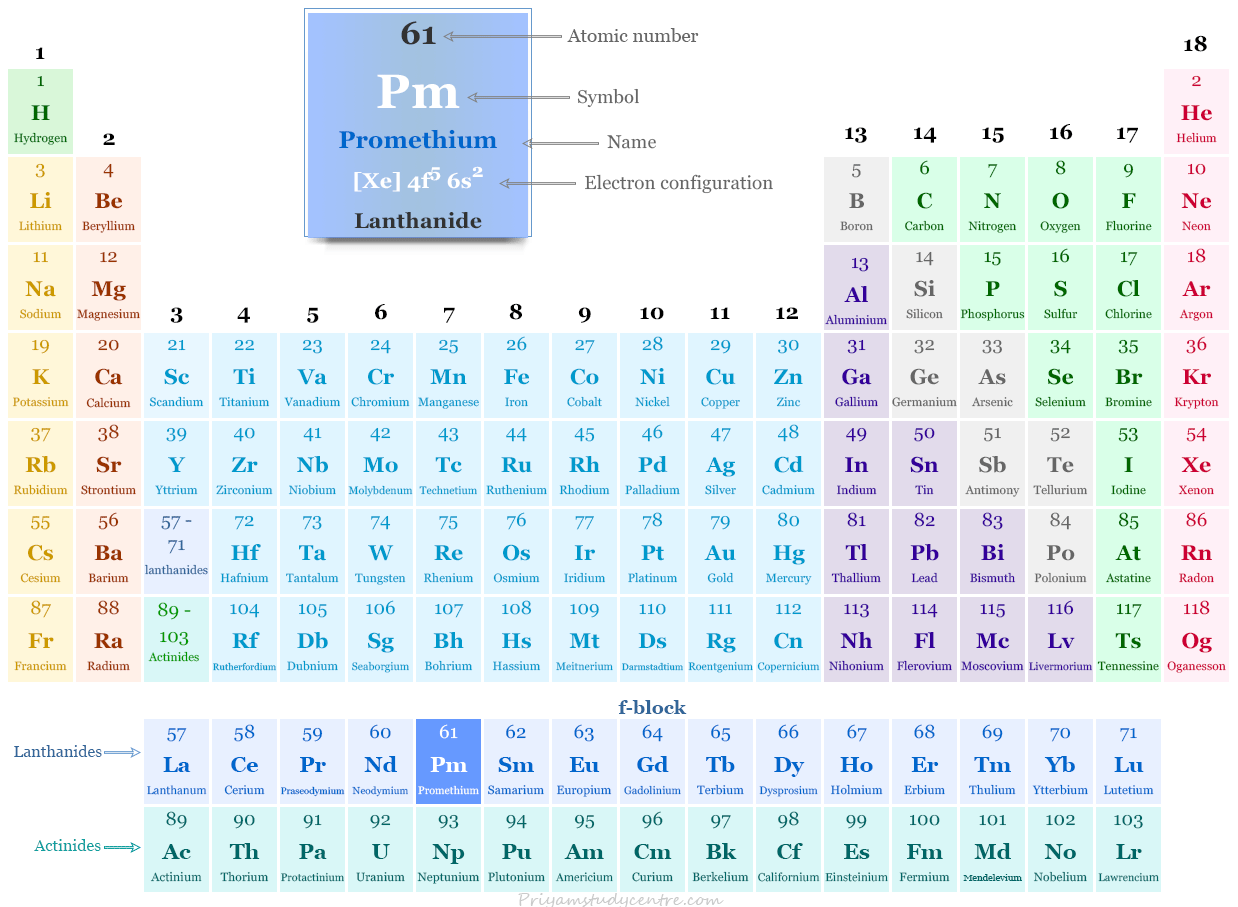
Promethium in the Periodic Table
The radioactive element promethium is placed with lanthanide or rare earth metal in the periodic table. It is an f-block element that lies between neodymium and samarium.
Chemical Properties
The chemical studies of promethium are incomplete due to its instability. The electron configuration for Pm3+ is [Xe] 4f4 and the color of the ionic solution is pink.
Only a few compounds of Pm are synthesized but they are not fully studied. The main Pm3+ compounds may include,
- Pm(OH)3 (light brown)
- Pm2O3 (yellow-white)
- PmCl3 (yellow)
- Pm(NO3)2 (pink)
- PmF3
- Pm2(C2O4)3,10H2O
- Pm2(SO4)3
Acidic solutions containing Pm3+ ions react with ammonia to form a gelatinous light-brown hydroxide, Pm(OH)3. It is insoluble in water.
Pm+3 is dissolved in hydrochloric acid to form a water-soluble yellow salt, PmCl3. Similarly, when dissolved in nitric acid, it forms promethium nitrate with the chemical formula Pm(NO3)3.
Isotopes
Promethium is the only lanthanide that has no stable or long-lived isotopes. The longest-lived isotope is promethium-145 with a half-life of 17.7 years. The primary decay products or Pm isotopes are neodymium and samarium isotopes.
Promethium-147 (half-life of 2.68 years) is the most common and useful isotope of lanthanide Pm. It is a beta-particle-emitting isotope. It can be separated from other rare earth fusion products by ion exchange chromatography.
Promethium-147 is used mainly in luminous paint and atomic batteries. Promethium-147 is also used in the nuclear industry to measure the thickness of the inner surface layer of graphite in the cladding tube where the nuclear fuel rod is placed in a nuclear reactor.
Facts About Promethium
- Promethium is the only radioactive element in the lanthanide series that has no stable isotopes.
- It is a cerium group of lanthanides that is chemically similar to that of neighboring elements neodymium and samarium.
- It is the only lanthanide element found in nature with a very trace amount.
- It was discovered in 1945 by USA chemists Jacob Marinsky, Lawrence Glendenin, and Charles Coryell but they did not announce their discovery until 1947.
- The chemical properties of Pm and its compounds cannot be fully studied due to the very small abundance of the metal.
- It has no biological role in the human body.
- The isotope Pm-147 can emit gamma rays during its beta decay. Therefore, it is dangerous for human health. The interactions with tiny quantities of Pm-147 are not hazardous if certain precautions are maintained.
Uses of Promethium
It is a very rare element. Therefore, promethium is used only for research purposes. Only the isotope, promethium-147 is used outside the laboratories. Neodymium-146 has been used for the production of 147Pm.
- The isotope 147Pm is used in luminous paint for some signal lights. Previously, radium-226 was used for the production of luminous paint. Presently it is replaced by promethium-147 and tritium due to nuclear safety reasons.
- Promethium-147 is used widely for making long-life atomic batteries for satellites or space probes. It may be made by small-scale promethium samples inserted into a semiconductor matrix to transform their beta emission into electric current.
- Pm-147 is an auxiliary source of heat or power for space probes and satellites.
- It is also used in thickness-measurement devices. The device is used mainly to measure the thickness of the inner surface layer of graphite in the cladding tube where the nuclear fuel rod is placed in a nuclear reactor.
- In medicine, promethium beta therapies may be used for the treatment of lumbosacral radiculitis.
- Promethium is applied in lasers that are used in satellite to submarine laser communication systems.