What is Neodymium?
Neodymium is a chemical element or rare earth metal in the periodic table with the symbol Nd and atomic number 60. It is a hard, silvery, slightly malleable f-block element that is the fourth member of the lanthanide series. It is an important component of neodymium magnets that are obtained by alloying with iron and boron. The strong permanent magnets of rare earth metal neodymium are used widely in many electronic devices such as mobile phones, microphones, loudspeakers, electronic musical instruments, and wind turbines. The rare earth metals praseodymium and neodymium were discovered in 1885 by the Austrian chemist Carl Auer von Welsbach. The name neodymium is obtained from the Greek words neos means new and didymos means twin. Commercially, neodymium compounds were used first for glass coloration.

Where is Neodymium Found?
It is the second most abundant of the rare-earth metals after lanthanum and cerium. It is almost the same abundant as copper. Neodymium is found commercially in two main ores such as monazite and bastnasite.
The rare earth mineral monazite is chemically quite inert and is mostly found in beach sands and river beds through weathering. The rare earth ore monazite is found mainly in China, the USA, Brazil, India, South Africa, Sri Lanka, Australia, and Malaysia. Another ore bastnasite occurs mainly in USA and China.
Isotopes
The lanthanide neodymium has five stable naturally occurring isotopes 142Nd, 143Nd, 145Nd, 146Nd, and 148Nd. 33 other radioactive isotopes may be synthesized by various artificial nuclear reactions. The radioactive isotopes 144Nd and 150Nd have very long half-lives.
The half-lives of remaining radioactive isotopes have less than that of twelve days. The majority of radioactive Nd isotopes have half-lives shorter than 70 seconds.
142Nd has been used for the production of short-lived thulium and ytterbium isotopes. 146Nd has been used for the production of 147Pm. Promethium-147 is used in luminous paint, atomic batteries, and thickness-measurement devices.
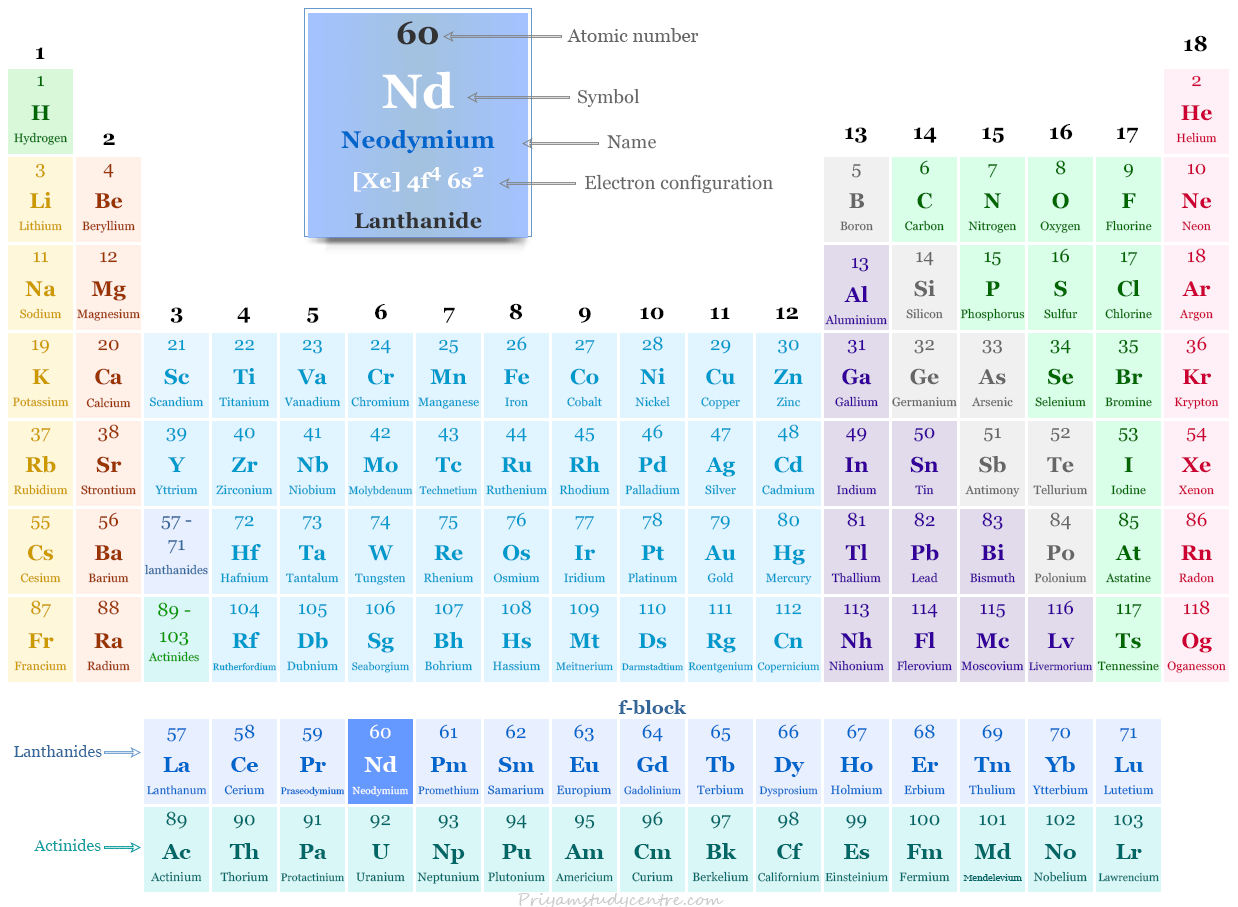
Neodymium in the Periodic Table
It is placed with lanthanides or f-block elements in the periodic table. Neodymium is a rare earth metal that lies between praseodymium and promethium.
Properties
It is the fourth member of the lanthanide series that forms a bright, silvery metallic luster. It is an active metal that is highly malleable and ductile. Neodymium is alloyed with other metals like iron and boron to make stable and powerful magnets that are used in various commercial equipment.
| Neodymium | |||
| Symbol | Nd | ||
| Discovery | Carl Auer von Welsbach in 1885 | ||
| Name derived from | The Greek word neos didymos means new twin | ||
| Common isotope | 60Nd142 | ||
| Oxidation states | +3, +2, +4 | ||
| CAS number | 7440-00-8 | ||
| Periodic properties | |||
| Atomic number | 60 | ||
| Relative atomic mass | 144.242 | ||
| Electron per cell | 2, 8, 18, 22, 8, 2 | ||
| Electronic Configuration | [Xe] 4f4 6s2 | ||
| Block | f-block | ||
| Group | Lanthanides | ||
| Period | 6 | ||
| Physical properties | |||
| State at 20 °C | Solid | ||
| Melting point | 1016 °C, 1289 K | ||
| Boiling point | 3074 °C, 3347 K | ||
| Molar heat capacity | 27.45 J mol−1 K−1 | ||
| Crystal structure | hexagonal close-packed (hcp) | ||
| Density | 7.01 g/cm3 | ||
| Heat of fusion | 7.14 kJ mol−1 | ||
| Heat of vaporization | 289 kJ mol−1 | ||
| Atomic properties | |||
| Atomic radius (non-bonded) | 2.39 Å | ||
| Covalent radius | 1.88 Å | ||
| Electronegativity | 1.14 (Pauling scale) | ||
| Electron affinity | Unknown | ||
| Ionization energy (kJ/mol) | 1st | 2nd | 3rd |
| 533.08 | 1034.32 | 2132.30 | |
Like other rare earth metals, neodymium may not serve any known biological function in the human body. It is not very toxic but neodymium dust and salts are very irritating to our eyes and mucous membranes, and moderately irritating to our skin.
Electron Configuration
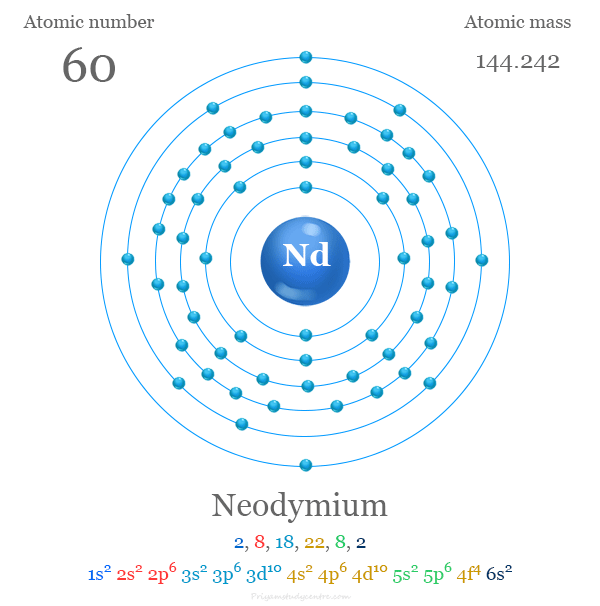
The 60 electrons of neodymium are arranged to show the electron configuration of [Xe] 4f4 6s2. Like other lanthanides, Nd commonly uses three valence electrons to show a +3 oxidation number or state. The remaining 4f electrons are strongly bound due to the greater stabilization of 4f orbitals in comparison to 5d or 6s.
Chemical Properties
Neodymium is a highly electropositive and active metal. The electrode potential value of Nd is comparable to that of alkaline earth metals. Nd acts as a strong reducing agent.
The compact metal is quite stable to dry air at ordinary temperatures but rapidly dull in a humid atmosphere. Neodymium metal tarnishes slowly in the air by the formation of an oxide layer. Like praseodymium, it also burns in oxygen at 150 °C to form Nd (III) oxide.
4 Nd + 3 O2 → 2 Nd2O3
Neodymium is attacked by acids like dilute sulfuric acid with the liberation of hydrogen gas. The acidic solutions contain Nd3+ ions that exist as [Nd(H2O)9]3+ complexes.
2 Nd + 3 H2SO4 → 2 Nd3+ + 3 SO4−2 + 3 H2
Chemical Compounds
Neodymium Hydroxide
The rare earth metal neodymium dissolves slowly in cold water but more rapidly in warm water with the liberation of hydrogen. It forms Nd (III) hydroxide with water.
2 Nd + 6 H2O → 2 Nd(OH)3 + 3 H2
Halides
Neodymium metal reacts with all the halogens like chlorine, fluorine, bromine, or iodine to form trihalides.
2 Nd + 3 F2 → 2NdF3 (violet)
2 Nd + 3 Cl2 → 2NdCl3 (mauve)
3 Br2 + 2 Nd → 2NdBr3 (violet)
2 Nd + 3 I2 → 2NdI3 (green)
Carbonate
Neodymium carbonate is a chemical compound with the chemical formula Nd2(CO3)2. It may be prepared by passing carbon dioxide in an aqueous solution of Nd(OH)3.
The carbonate is insoluble in water but dissolves in acids with the liberation of carbon dioxide.
Sulfate
It is a salt of the rare-earth metal neodymium with the chemical formula Nd2(SO4)3. Neodymium sulfate is obtained by dissolving Nd (III) oxide in sulfuric acid.
Nd2O3 + 3 H2SO4 → Nd2(SO4)3 + 3 H2O
It forms multiple hydrates like octa-, penta-, and dihydrate but the octahydrate form is the most common. It is used in glass for making extremely powerful lasers.
Acetate
Neodymium acetate is a salt that contains one Nd+3 ion and three acetate anions with the chemical formula of Nd(CH3COO)3. Nd acetate is a purple solid that is soluble in water.
It can be formed when acetic acid reacts with neodymium oxide, hydroxide, or carbonate.
6 CH3COOH + Nd2O3 → 2 Nd(CH3COO)3 + 3 H2O
3 CH3COOH + Nd(OH)3 → Nd(CH3COO)3 + 3 H2O
It may be used for making ultra-high purity compounds, catalysts, and nanoscale materials. Neodymium acetate is a substitute for uranyl acetate in electron microscopy. It may also be used in glass, crystal, capacitors, and lenses for welding goggles.
Uses of Neodymium
Commercially, neodymium uses mainly for making powerful magnets, for coloration of glasses, making powerful lasers, making fertilizers, etc. The most common use of Nd metal is given below,
Neodymium Magnets
A neodymium magnet is the most widely used commercial magnet of rare earth metals. It is a permanent magnet that is made from an alloy of neodymium, iron, and boron to form a tetragonal crystalline solid with the chemical formula Nd2Fe14B.
The strong permanent magnets of rare earth metal neodymium are used widely in many electronic devices such as
- mobile phone speakers
- computer hard disks
- microphones and loudspeakers
- electronic musical instruments
- wind turbines
It is also used in mechanical e-cigarette firing switches, locks for doors, magnetic bearings and couplings, NMR spectrometers, etc.
Neodymium Glass
Commercially, pure neodymium was used first in glass coloration. The color of the glasses changes under different lighting conditions. It shows reddish-purple under daylight or yellow incandescent light but blue under white fluorescent lighting or greenish under trichromatic lighting.
Neodymium glass is obtained by mixing neodymium oxide (Nd2O3) in the glass melt. The red color of glasses may be produced by combining gold or selenium. The most common use of these glasses may include,
- It is used widely for the production of natural light in incandescent light bulbs.
- It is also used in automobile rear-view mirrors to reduce glare at night.
- The Nd glass is used in astronomical work to produce sharp bands by which spectral lines may be calibrated.
- The rare earth metal neodymium can be used to remove the green color caused by iron contaminants from the glass.
- Didymium is a natural mixture of Pr and Nd. It is used for making protective glasses that absorb ultraviolet radiation.
- Nd glass is used for glass blowing and welding work.
Neodymium Lasers
They are liquid lasers obtained from certain transparent materials with a small concentration of neodymium ions. These layers are very powerful and offer many technical advantages.
- Nd:YAG (yttrium aluminium garnet): It is a crystalline solid that is used in solid-state lasers.
- Nd:YAP (yttrium aluminium perovskite): It is a laser that has various clinical applications.
- Nd:YLF (yttrium lithium fluoride): It is a lasing medium for arc lamp-pumped and diode-pumped solid-state lasers.
- Nd:YVO4 (yttrium orthovanadate): It is a crystalline material obtained by adding Nd ions to yttrium orthovanadate.
- Nd:glass: It is a laser that is obtained by dipping Nd+3 in glasses.
In Fertilizer
In agriculture, lanthanide compounds such as Pr and Nd compounds are used as insecto fungicides and trace elements in fertilizers. They are frequently used for making fertilizers in China.
In Transmission Electron Microscopy
Uranyl acetate is a radioactive and toxic substance that has been used as a standard contrasting agent in transmission electron microscopy (TEM) for decades. It causes various health hazards or radioactive pollution.
Presently, neodymium acetate or platinum blue may be used as a standard contrasting agent instead of uranyl acetate in transmission electron microscopy.
Neodymium Price
Neodymium is the second most abundant rare earth metal after lanthanum and cerium. The metal Nd is used widely for making magnets and the coloration of glasses.
In 2020, the price of the rare earth metal oxide neodymium oxide was 49,763 US dollars per metric ton. The price of neodymium magnets is relatively higher than that of other magnets due to the high price of rare earth metal Nd.