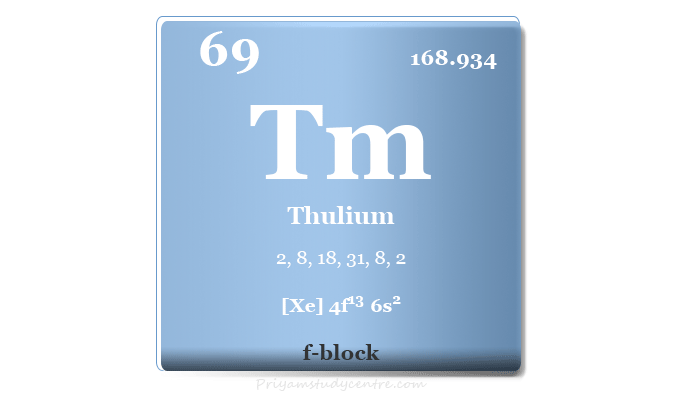
Thulium Metal
Thulium is a chemical element or rare earth metal in the periodic table with the symbol Tm and atomic number 69. It is a second rarer lanthanide after radioactively unstable samarium. Like other lanthanides, thulium most commonly shows a +3 oxidation number or state but the +2 oxidation state of the metal is also stable. Thulium is rarely used in X-ray devices and solid-state lasers due to its high price. Like scandium, it is used in arc lighting for the production of green emission spectra. Recent reports show that thulium optical fiber laser provides an impressive level of performance in laser lithotripsy.
Thulium metal was discovered in 1879 by Swedish chemist Per Teodor Cleve from the mineral erbia which is a source of other rare earth element holmium. It was named from the word Thule, the ancient name for Scandinavia.
Where is Thulium Found?
A pure form of thulium is never found in nature but it is found in small quantities with other rare earth minerals such as monazite and bastnasite. It is the second rarest lanthanide after samarium. The chief ores of the metal are mined in the regions of China, the US, Brazil, India, Sri Lanka, and Australia.
Like other rare earth metals, it can be extracted or separated by ion exchange chromatography and solvent extraction process. The metal is also produced by reducing the anhydrous fluoride with calcium or reducing thulium oxide with lanthanum.
Isotopes
Naturally occurring thulium contains one stable isotope, 169Tm. Thirty-four radioactive isotopes and 26 nuclear isomers have been prepared by various artificial nuclear reactions.
The majority of these isotopes have half-lives shorter than a minute. The primary radioactive decay mode of Tm isotopes is electron capture or beta decay.
Properties
Thulium is a silvery metal that is fairly stable in the air and reacts slowly in water but more quickly in acids. It is a soft metal that can be easily cut with a knife.
| Thulium | |||
| Symbol | Tm | ||
| Discovery | Per Teodor Cleve in 1879 | ||
| Name derived from | From Thule, the ancient name for Scandinavia | ||
| Common isotope | 69Tm169 | ||
| Oxidation states | +3, +2 | ||
| CAS number | 7440-30-4 | ||
| Periodic properties | |||
| Atomic number | 69 | ||
| Relative atomic mass | 168.934 | ||
| Electron per cell | 2, 8, 18, 31, 8, 2 | ||
| Electronic configuration | [Xe] 4f13 6s2 | ||
| Block | f-block | ||
| Group | Lanthanides | ||
| Period | 6 | ||
| Physical properties | |||
| State at 20 °C | Solid | ||
| Melting point | 1545 °C, 1818 K | ||
| Boiling point | 1950 °C, 2223 K | ||
| Molar heat capacity | 27.03 J mol−1 K−1 | ||
| Crystal structure | hexagonal close-packed (hcp) | ||
| Density | 9.32 g/cm3 | ||
| Heat of fusion | 16.84 kJ mol−1 | ||
| Heat of vaporization | 191 kJ mol−1 | ||
| Atomic properties | |||
| Atomic radius (non-bonded) | 2.27 Å | ||
| Covalent radius | 1.77 Å | ||
| Electronegativity | 1.25 (Pauling scale) | ||
| Electron affinity | 99.283 kJ mol−1 | ||
| Ionization energy (kJ/mol) | 1st | 2nd | 3rd |
| 596.69 | 1162.65 | 2284.77 | |
Thulium in the Periodic Table
The rare earth metal thulium is placed in the f-block of the periodic table. It is a lanthanide that lies between erbium and ytterbium.
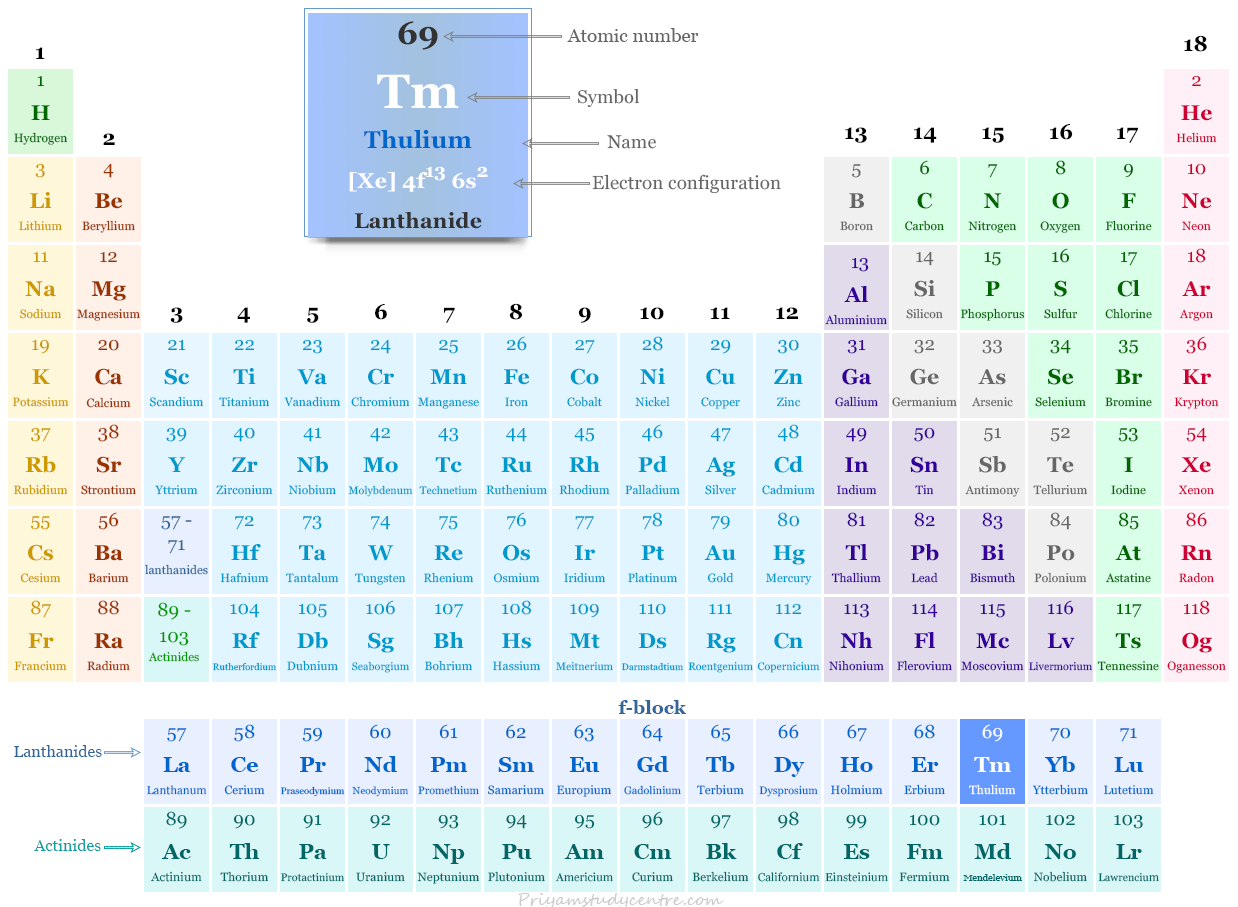
Electron Configuration of Thulium
The 69 electrons in thulium are distributed in different energy levels or orbitals to show the following electron configuration given below the picture,
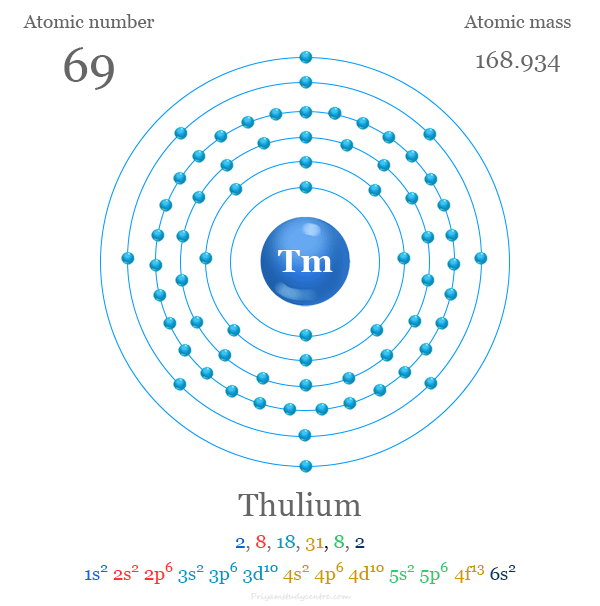
Chemical Properties
Like other lanthanides, the most common oxidation number or state of thulium is +3 and it exists as a Tm3+ ion in solution. It reacts with various metallic and non-metallic elements forming a range of binary compounds.
Thulium oxide is formed by heating metal in oxygen or thermal decomposition of Tm(OH)3.
4 Tm + 3 O2 → 2 Tm2O3
Thulium stands far above hydrogen in the electrochemical series. Therefore, it reacts slowly with cold water and rapidly with hot water by the liberation of hydrogen and the formation of Tm (III) hydroxide.
2 Tm + 6 H2O → 2 Tm(OH)3 + 3 H2
The hydroxide is precipitated as a gelatinous precipitate from an aqueous solution by the addition of ammonia or diluted alkali to form a soluble salt of Tm+3 ion.
It forms dihalides when reacts with halogens like fluorine, chlorine, bromine, and iodine.
2 Tm + 3 X2 → 2 TmX3 (X = F, Cl, Br, I)
It is attacked by mineral acids like sulfuric acid, nitric acid, and hydrochloric acid with the liberation of hydrogen gas. In the presence of dilute sulfuric acid, thulium forms a solution that contains the pale green Tm (III) ions.
2 Tm + 3 H2SO4 → 2 Tm3+ + 3 SO4−2 + 3 H2
Facts About Thulium
- The least abundant rare earth metal thulium is difficult to extract or purify from their ores.
- The most common oxidation state of Tm is +3 but the +2 state of the metal is also stable.
- There are not many uses for thulium and its compounds due to its rarity of appearance and expensive price.
- After the development of the ion exchange chromatography technique, the price of the metal becomes decreased.
- In solid-state laser technology, holmium-chromium-thulium garnet produces high-efficiency lasers. These lasers are useful tools for surgical applications due to their strength and wavelength.
- Like scandium, it is used in arc lighting for the production of green emission lines that are not emitted by other chemical elements.
- It is not a toxic metal with any known biological function on human beings.
Uses of Thulium
- An isotope of thulium obtained from irradiation of a nuclear power reactor uses as a source of radiation for portable X-ray devices.
- It is an important component for high-efficiency lasers. These lasers are used in the fields of medical, astronomical, and military.
- Like yttrium, it is used for making high-temperature superconductors.
- It is a component of ferrites and ceramic magnetic materials that are used in microwave equipment.
- The element is used for anticounterfeiting markings in euro banknotes due to its blue fluorescence properties.
- Thulium is used in arc lighting because it provides an unusual spectrum like scandium.
- The Holmium:yttrium-aluminum-garnet laser has been used for laser lithotripsy over the last 20 years but recent reports show that thulium fiber laser (TFL) lithotripter provides an impressive level of performance.