Cerium Metal
Cerium is a chemical element or rare earth metal in the periodic table with the symbol Ce and atomic number 58. It is a soft, ductile, and silvery-white metal that lies with f-block elements or lanthanides. The metal cerium is one of the most abundant of the rare-earth metals that makes up to 66 ppm of the Earth’s crust. The value is just behind that of copper (68 ppm) and it is more abundant than some metals like lead and tin. It is a silvery white, soft, and malleable metal with high electrical conductance. Cerium metal and its compounds may be used to polish glasses, catalytic converters, ferrocerium lighters, and light-emitting diodes to produce white light for commercial LED light.

In 1803, the lanthanide cerium was discovered by Swedish chemists Jöns Jakob Berzelius and Wilhelm Hisinger and independently by German chemist Martin Heinrich Klaproth in the same year. It was isolated first in 1839 by Carl Gustaf Mosander. It is named after the asteroid Ceres or named after the Roman goddess of agriculture.
Where is Cerium Found?
Cerium is one of the most abundant of the rare-earth metals among all the lanthanides. It is found in several minerals such as allanite or orthrite, monazite, bastnasite, cerite and samarskite. Cerium is a class of rare earth metals but the abundance of Ce in Earth’s crust is not rare.
India, Brazil, and Southern California are the largest depositors and producers of rare earth metal cerium.
Isotopes
Naturally occurring cerium contains 4 stable isotopes 136Ce, 138Ce, 140Ce, and 142Ce. The half-life of the most stable isotopes is
144Ce: half-life of 284.9 days
139Ce: half-life of 137.6 days
141Ce: half-life of 32.5 days
There are 35 radioactive isotopes with mass numbers ranging from 119 to 157. These isotopes are synthetic and obtained by various nuclear reactions. Many of these isotopes occur in the nuclear fission process of uranium.
All the other radioactive isotopes have a half-life of fewer than four days. Many radioactive isotopes have a half-life of fewer than 10 minutes. The radioactive isotope, 142Ce is used in analytical chemistry to study co-precipitation and chromatography separation.
Properties
Cerium is a silvery white, soft, and malleable metal with high electrical conductance. The appearance of Ce is similar to that of silver metal.
| Cerium | |||
| Symbol | Ce | ||
| Discovery | Jöns Jacob Berzelius and Wilhelm Hisinger in 1803 | ||
| Name derived from | It is named after the asteroid, Ceres or named after the Roman goddess of agriculture | ||
| Common isotope | 58Ce140 | ||
| Oxidation states | +4, +3 | ||
| CAS number | 7440-45-1 | ||
| Periodic properties | |||
| Atomic number | 58 | ||
| Relative atomic mass | 140.116 | ||
| Electron per cell | 2, 8, 18, 19, 9, 2 | ||
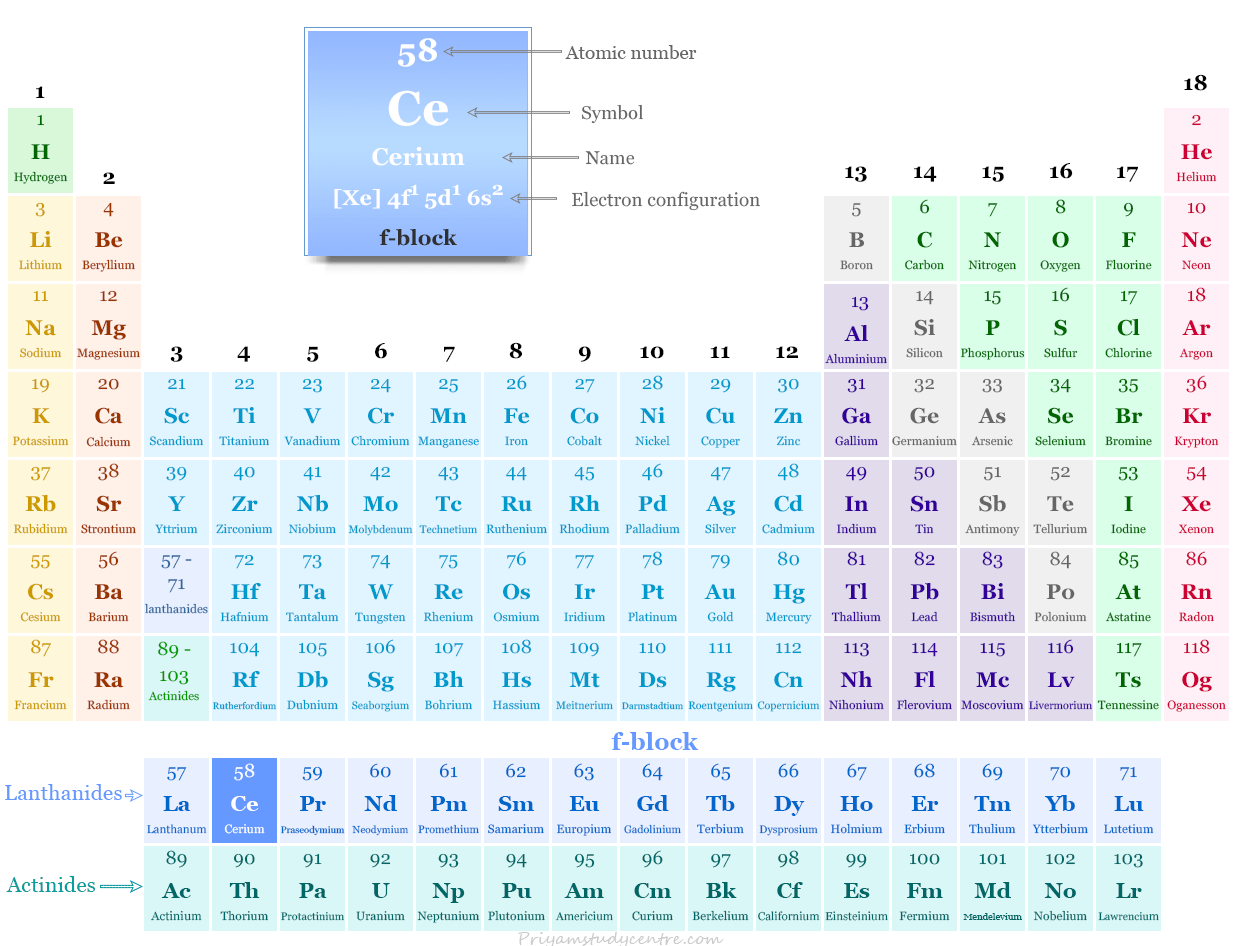
| Electronic Configuration | [Xe] 4f1 5d1 6s2 | ||
| Block | f-block | ||
| Group | Lanthanides | ||
| Period | 6 | ||
| Physical properties | |||
| State at 20 °C | Solid | ||
| Melting point | 799 °C, 1072 K | ||
| Boiling point | 3443 °C, 3716 K | ||
| Molar heat capacity | 26.94 J mol−1 K−1 | ||
| Crystal structure | β-Ce | γ-Ce | |
| double hexagonal close-packed (dhcp) | face-centered cubic (fcc) | ||
| Density | 6.77 g/cm3 | ||
| Electrical resistivity | 828 nΩ m | ||
| Atomic properties | |||
| Atomic radius (non-bonded) | 2.42 Å | ||
| Covalent radius | 1.84 Å | ||
| Electronegativity | 1.12 (Pauling scale) | ||
| Electron affinity | 62.72 kJ mol−1 | ||
| Ionization energy (kJ/mol) | 1st | 2nd | 3rd |
| 534.40 | 1046.87 | 1948.81 | |
Electron Configuration
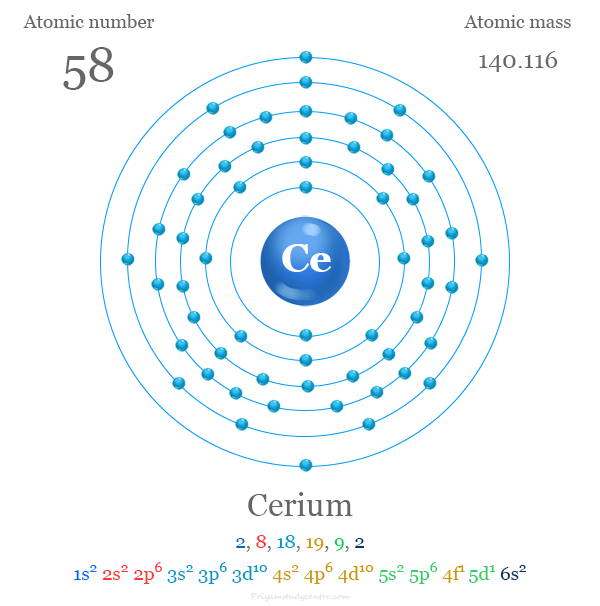
The general electronic configuration of the lanthanide elements is [Xe] 4fn 6s2. Cerium is a lanthanide whose electronic configuration is outside this generalization.
The 58 electrons of Ce are arranged in the configuration of [Xe] 4f1 5d1 6s2. In Ce, the effective nuclear charge is not sufficient to stabilize the general configuration [Xe] 4f2 6s2 compared to [Xe] 4f1 5d1 6s2.
The 4f, 5d, and 6s energy levels are very close to each other. Therefore, the transfer of one electron to the 5d shell is occurring due to strong interelectronic repulsion in the compact 4f shell.
Oxidation States
The principal oxidation state of all lanthanides is +3. The element adjacent to lanthanum (4f0), gadolinium (4f7), and lutetium (4f14) show other oxidation states.
Cerium has four outer electrons or valence electrons in 4f, 5d, and 6s energy levels. After losing these four electrons, it forms a Ce+4 ion. The Ce+4 ion is stabilized by an empty f-shell configuration.
Cerium in the Periodic Table
It is placed in the f-block of the periodic table. Cerium lies between lanthanum and praseodymium in the lanthanides series.
Chemical Reactions
It burns readily in oxygen at 150 °C to form the pale-yellow Ce (IV) oxide or ceria.
Ce + O2 → CeO2
It reacts with hydrogen to form stable CeH2 and CeH3. The hydrides of cerium are non-stoichiometric in nature.
On heating, cerium reacts with a number of non-metals like nitrogen, sulfur, carbon, silicon, phosphorus, hydrogen, etc. On heating with carbon, it forms carbide CeC2. The carbide CeC2 shows metallic conductivity.
Cerium is a highly electropositive rare earth metal. Therefore, it reacts with water to form Ce (III) hydroxide and hydrogen gas. The rate of production increases with increasing temperature.
2 Ce + 6 H2O → 2 Ce(OH)3 + 3 H2
It dissolves readily in dilute sulfuric acid to form a solution that contains colorless Ce3+ ions.
2 Ce + 3 H2SO4 → 2 Ce3+ + 3 SO2−4 + 3 H2
Interesting Facts About Cerium
- Cerium is a lanthanide whose electronic configuration is outside the general electron configuration. The electron configuration of Ce is [Xe] 4f1 5d1 6s2.
- It shows a stable +4 oxidation state or number due to an empty f-shell configuration of Ce+4 ions.
- It is a highly electropositive and reactive rare earth metal. The electropositivity of heavier rare earth elements is comparable to calcium and scandium to aluminum.
- The compact metal is quite stable to dry air at ordinary temperatures but rapidly becomes dull in the humid atmosphere.
- Powder cerium is pyrophoric in nature. It burns in the air to form oxide and nitride.
- Chelating ligands like ethylenediamine tetraacetic acid (EDTA) and critic acid form soluble complexes with cerium. It is useful in their separation by ion exchange chromatography.
Chemical Compounds
The rare earth metal cerium shows +3 and +4 oxidation states. The details about oxide, fluoride, and sulfate compounds of cerium are given below the topics,
Cerium (IV) Oxide
Cerium (IV) oxide or ceria is a pale yellow-white powder with the chemical formula CeO2. It is an important commercial substance and an intermediate that is used widely for the production of other elements from their ores.
Cerium oxide (CeO2) is an industrial chemical substance that is used mainly for polishing glasses and making protective transparent glasses. It is also used in mixed conductors, gas tungsten Arc welding, biochemical research, photocatalysis, fuel cells, and biological antioxidants.
Fluoride
The fluoride compounds of cerium are obtained in +3 and +4 oxidation states. Ce (III) fluoride is an ionic compound of rare earth metal cerium and fluorine. Ce (IV) fluoride is a strong oxidant that forms a white crystalline solid.
CeF4 may be prepared by reaction of CeF3 with fluorine at room temperature. It is stable up to 800 °C.
Ce+4 + e → Ce+3
Cerium Sulfate
The rare earth metal cerium formed sulfate compounds in +3 and +4 oxidation states.
Cerium (III) sulfate is an inorganic compound with the chemical formula Ce2(SO4)3. Anhydrous Ce (III) sulfate is a hygroscopic white solid that begins to decompose above 600 °C.
Cerium (IV) sulfate is a strong oxidizer with the chemical formula Ce(SO4)2. It is obtained in anhydrous and various hydrated forms.
The elemental chlorine is formed when ceric sulfate is added to dilute hydrochloric acid. In analytical chemistry, cerium (IV) sulfate is used as an oxidant in titrations in place of permanganate or dichromate. In acetic acid solution, it oxidizes aldehydes and ketones at the α-carbon atom.
Uses of Cerium
- It is used in metallothermic reactions due to its extraordinary reducing properties. Cerium is a stronger reducing agent than aluminum. Lanthanide-thermic processes yield sufficiently pure niobium, zirconium, iron, cobalt, nickel, manganese, tungsten, etc.
- It is the key component of alloy mischmetal that is useful for cigarette lighter flints.
- Ceria or cerium oxide (CeO2) is the most widely used compound of the metal. It is used as an abrasive for polishing glasses. The mixture of CeO2 and other metal oxides has been used mainly for polishing glasses.
- Approximately 1% of CeO2 is used for manufacturing protective transparent glasses. It is used in nuclear technology to block harmful radiation.
- CeS is used in the manufacture of special types of crucibles that are used for melting metals up to 1800 °C.
- For making paints, cerium molybdate gives light yellow colour, and tungstate form gives greenish blue color.
- Salts of Ce are used as activators of luminophore. It is used for making gas mantles, and LED lights, and painting the screen of cathode tubes.
- In medicinal chemistry, cerium salts are used for the treatment of vomiting and sea sickness.
- In analytical chemistry, cerium sulfate is used as an oxidizing agent in volumetric analysis.
- The radioactive isotope, 142Ce is used to study co-precipitation and separation by chromatography.
- Cerium phosphate is used as a chemical catalyst in petroleum creaking.
Cerium Price
It is the most abundant rare earth metal. Therefore, the price of cerium and its compounds is less than that of other heavier rare earth metals.