Ytterbium Metal
Ytterbium is a chemical element or rare earth metal in the periodic table with the symbol Yb and atomic number 70. Pure metal is a soft, malleable, and ductile chemical element that displays a bright silvery luster. The density, melting, and boiling point of ytterbium differ from other rare earth metals due to its closed-shell electronic configuration. Ytterbium is a useful component for making the world’s most stable atomic clock. The compound of metal, ytterbium oxide (Yb2O3) is a useful component for making glasses, laser system, optical fibers, special alloys, and dielectric ceramic materials. Much progress has been observed in optical fiber technology after using a ytterbium-doped optical laser.
Ytterbium was discovered in 1879 by the Swiss chemist Jean Charles Galissard de Marignac while examining samples of gadolinite. He found a new component from gadolinite known as erbia. He named it Ytterbia for Ytterby means the Swedish village near where the new component of erbium was found.
Where is Ytterbium Found?
Ytterbium is the 44th most abundant element in the Earth’s crust and one of the more common rare earth metals found with other rare-earth minerals.
The most common and commercially important mineral of the metal is monazite. The element is also found in the minerals euxenite and xenotime.
The main mining regions of ytterbium metal are China, the United States, Brazil, India, Sri Lanka, and Australia. It can be extracted and separated from other rare earth minerals by ion exchange chromatography and solvent extraction process.
Isotopes
Naturally occurring ytterbium is a mixture of seven stable isotopes with atomic mass ranging from 168 to 176. The most common isotope is ytterbium-174 which occurs in about 31.8 percent of the natural abundance. At least 27 radioactive isotopes have been obtained by various nuclear reactions.
Most of these radioactive isotopes have half-lives of less than 20 minutes. The primary radioactive decay mode of ytterbium isotopes is electron capture or beta decay. It also displays 12 meta-stable isotopes with the most stable being ytterbium-169m.
Properties
Soft, silvery Yb oxidizes slowly in the air by the formation of a protective surface layer. The finely divided ytterbium powdered will ignite in the air.
| Ytterbium | |||
| Symbol | Yb | ||
| Discovery | Jean Charles Galissard de Marignac in 1878 | ||
| Name derived from | From the mineral, Ytterby found in Sweden | ||
| Common isotopes | 70Yb172, 70Yb173, and 70Yb174 | ||
| Oxidation states | +3, +2 | ||
| CAS number | 7440-64-4 | ||
| Periodic properties | |||
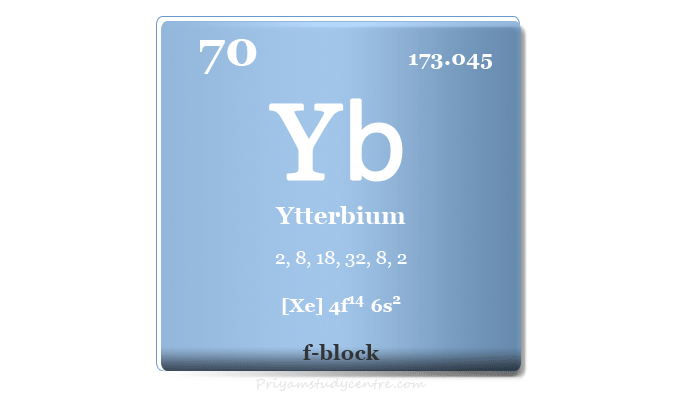
| Atomic number | 70 | ||
| Relative atomic mass | 173.045 | ||
| Electron per cell | 2, 8, 18, 32, 8, 2 | ||
| Electronic Configuration | [Xe] 4f14 6s2 | ||
| Block | f-block | ||
| Group | Lanthanides | ||
| Period | 6 | ||
| Physical properties | |||
| State at 20 °C | Solid | ||
| Melting point | 824 °C, 1097 K | ||
| Boiling point | 1196 °C, 1469 K | ||
| Molar heat capacity | 26.74 J mol−1 K−1 | ||
| Crystal structure | face-centered cubic (fcc) | ||
| Density | 6.90 g/cm3 | ||
| Heat of fusion | 7.66 kJ mol−1 | ||
| Heat of vaporization | 129 kJ mol−1 | ||
| Atomic properties | |||
| Atomic radius (non-bonded) | 2.26 Å | ||
| Covalent radius | 1.78 Å | ||
| Electronegativity | Unknown | ||
| Electron affinity | −1.93 kJ mol−1 | ||
| Ionization energy (kJ/mol) | 1st | 2nd | 3rd |
| 603.43 | 1174.80 | 2416.96 | |
Ytterbium in the Periodic Table
The rare earth metal ytterbium is placed with f-block elements in the periodic table. It is a lanthanide that lies between thulium and lutetium.
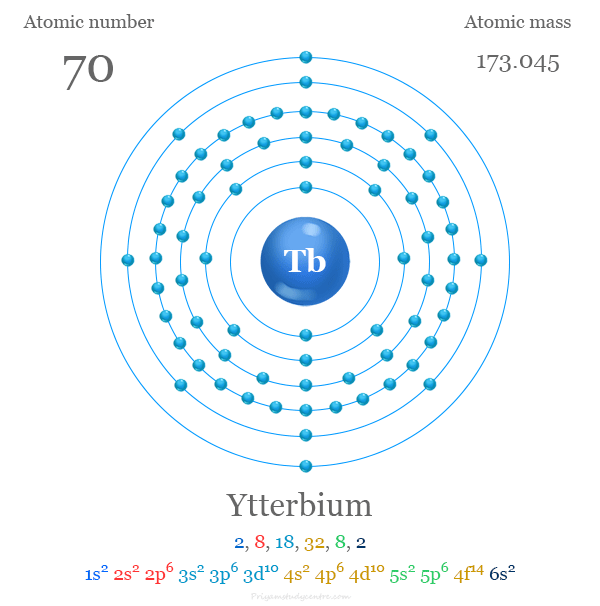
Electron Configuration of Ytterbium
The 70 electrons of the ytterbium atom are distributed in different energy levels to show the following electronic configuration,
Chemical Properties
Like other lanthanides, the most common oxidation number or state of ytterbium is +3 but the +2 oxidation state is also stable due to the f14 configuration of the Yb+2 ion. It reacts with hydrogen to form various non-stoichiometric hydride compounds.
Ytterbium is a highly reactive and electropositive rare earth metal that is stable in dry air at ordinary temperatures but rapidly dull in humid atmospheres. It readily reacts with oxygen to form ytterbium (III) oxide Yb2O3.
4 Yb + 3 O2 → 2 Yb2O3
Ytterbium stands far above hydrogen in the electrochemical series. Therefore, it reacts slowly with cold water and rapidly with hot water to liberate hydrogen and the formation of Yb (III) hydroxide.
2 Yb + 6 H2O → 2 Yb(OH)3 + 3 H2
It forms both dihalides and trihalide compounds when reacts with halogens like fluorine, chlorine, bromine, and iodine.
2 Yb + 3 X2 → 2 YbX3 (X = F, Cl, Br, I)
The dihalides are oxidized to the trihalides at room temperature. However, disproportionate to the trihalides and metallic Yb at high temperatures.
3 YbX2 → 2 YbX3 + Yb (X = F, Cl, Br, I)
It is readily dissolved by strong mineral acids like sulfuric acid, nitric acid, or hydrochloric acid. In dilute sulfuric acid, it forms a solution that contains the colorless Yb+3 ions which exist as nonahydrate complexes. It dissolves in ammonia to form blue electride salts.
2 Yb + 3 H2SO4 + 18 H2O → 2 [Yb(H2O)9]3+ + 3 SO4−2 + 3 H2
Facts About Ytterbium
- Ytterbium is a bright, shiny silver metal that is more reactive than the other lanthanide elements. Therefore, it is generally stored in sealed containers to save it from oxygen and humid atmospheres.
- The valence electron configuration of the Yb atom at +2 state is [Xe] 4f14 with a fully filled f-shell. Therefore, the +2 state of the metal is relatively stable due to the fully filled f-shell configuration.
- Colurless aqueous solution contains only Yb+3 because the yellow-green Yb+2 ion is a very strong reducing agent which decomposes water.
- Pure metal can be produced after the discovery of ion exchange chromatography.
- The compound of metal, ytterbium oxide (Yb2O3) is used for making glass, laser system, optical fiber, special alloys, and dielectric ceramic materials.
- It is found principally in the mineral monazite and extracted or separated by ion exchange chromatography and solvent extraction process.
- The price of the ytterbium metal is relatively more stable than the other rare earth elements.
- The low toxic Yb does not play any biological role in humans and other animals.
- Metallic ytterbium powdered causes fire in contact with air.
- It is used for replacing many too-toxic and polluting industrial catalysts.
Uses of Ytterbium
- It is used for making the world’s most stable atomic clock. A large number of atoms are the key factor for the high stability of atomic clocks.
- The Yb (III) ion or ytterbium oxide is a doping material that is used for making a solid-state laser and double-clad fiber laser.
- Huge progress has been observed in optical fibers technology after the discovery of ytterbium-doped optical fibers.
- It is a doping agent to improves grain refinement and mechanical strength of stainless steel.
- Like other rare earth metals, it can be used for making ceramic capacitors and other electronic devices,
- It is used as an industrial chemical catalyst for controlling various industrial processes. It has been used to replace other industrial catalysts which are too toxic and polluting.
- Presently, ytterbium metal and its oxide is used for making memory devices, and tunable lasers.